Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics
- PMID: 19306895
- PMCID: PMC2709600
- DOI: 10.1016/j.pharmthera.2009.02.009
Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics
Abstract
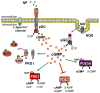
Cyclic guanosine 3',5'-monophosphate (cGMP) mediates a wide spectrum of physiologic processes in multiple cell types within the cardiovascular system. Dysfunctional signaling at any step of the cascade - cGMP synthesis, effector activation, or catabolism - have been implicated in numerous cardiovascular diseases, ranging from hypertension to atherosclerosis to cardiac hypertrophy and heart failure. In this review, we outline each step of the cGMP signaling cascade and discuss its regulation and physiologic effects within the cardiovascular system. In addition, we illustrate how cGMP signaling becomes dysregulated in specific cardiovascular disease states. The ubiquitous role cGMP plays in cardiac physiology and pathophysiology presents great opportunities for pharmacologic modulation of the cGMP signal in the treatment of cardiovascular diseases. We detail the various therapeutic interventional strategies that have been developed or are in development, summarizing relevant preclinical and clinical studies.
Figures

References
-
- Abraham WT, Lowes BD, Ferguson DA, Odom J, Kim JK, Robertson AD, Bristow MR, Schrier RW. Systemic hemodynamic, neurohormonal, and renal effects of a steady-state infusion of human brain natriuretic peptide in patients with hemodynamically decompensated heart failure. J Card Fail. 1998;4:37–44. - PubMed
-
- Ackermann U, Irizawa TG, Milojevic S, Sonnenberg H. Cardiovascular effects of atrial extracts in anesthetized rats. Can J Physiol Pharmacol. 1984;62:819–826. - PubMed
-
- Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. - PubMed
-
- Ammendola A, Geiselhoringer A, Hofmann F, Schlossmann J. Molecular determinants of the interaction between the inositol 1,4,5-trisphosphate receptor-associated cGMP kinase substrate (IRAG) and cGMP kinase Ibeta. J Biol Chem. 2001;276:24153–24159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

