Friends and foes in synaptic transmission: the role of tomosyn in vesicle priming
- PMID: 19307030
- PMCID: PMC2713869
- DOI: 10.1016/j.tins.2009.01.004
Friends and foes in synaptic transmission: the role of tomosyn in vesicle priming
Abstract
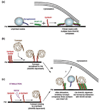
Priming is the process by which vesicles become available for fusion at nerve terminals and is modulated by numerous proteins and second messengers. One of the prominent members of this diverse family is tomosyn. Tomosyn has been identified as a syntaxin-binding protein; it inhibits vesicle priming, but its mode of action is not fully understood. The inhibitory activity of tomosyn depends on its N-terminal WD40-repeat domain and is regulated by the binding of its SNARE motif to syntaxin. Here, we describe new physiological information on the function of tomosyn and address possible interpretations of these results in the framework of the recently described crystal structure of the yeast tomosyn homolog Sro7. We also present possible molecular scenarios for vesicle priming and the involvement of tomosyn in these processes.
Figures
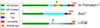

References
-
- Becherer U, Rettig J. Vesicle pools, docking, priming, and release. Cell Tissue Res. 2006;326:393–407. - PubMed
-
- Richmond J, Broadie K. The synaptic vesicle cycle: exocytosis and endocytosis in Drosophila and C. elegans. Curr Opin Neurobiol. 2002;12:499–507. - PubMed
-
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. - PubMed
-
- Jahn R, Scheller RH. SNAREs-engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

