Calcium homeostasis, selective vulnerability and Parkinson's disease
- PMID: 19307031
- PMCID: PMC4831702
- DOI: 10.1016/j.tins.2009.01.006
Calcium homeostasis, selective vulnerability and Parkinson's disease
Abstract
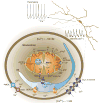
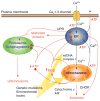
Parkinson's disease (PD) is a common neurodegenerative disorder of which the core motor symptoms are attributable to the degeneration of dopamine (DA) neurons in the substantia nigra pars compacta (SNc). Recent work has revealed that the engagement of L-type Ca(2+) channels during autonomous pacemaking renders SNc DA neurons susceptible to mitochondrial toxins used to create animal models of PD, indicating that homeostatic Ca(2+) stress could be a determinant of their selective vulnerability. This view is buttressed by the central role of mitochondria and the endoplasmic reticulum (linchpins of current theories about the origins of PD) in Ca(2+) homeostasis. Here, we summarize this evidence and suggest the dual roles had by these organelles could compromise their function, leading to accelerated aging of SNc DA neurons, particularly in the face of genetic or environmental stress. We conclude with a discussion of potential therapeutic strategies for slowing the progression of PD.
Figures
References
-
- de Lau LM, et al. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology. 2004;63:1240–1244. - PubMed
-
- Dorsey ER, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. - PubMed
-
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966;18:925–964. - PubMed
-
- Riederer P, Wuketich S. Time course of nigrostriatal degeneration in parkinson’s disease. A detailed study of influential factors in human brain amine analysis. J Neural Transm. 1976;38:277–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

