Cytolethal distending toxin promotes Helicobacter cinaedi-associated typhlocolitis in interleukin-10-deficient mice
- PMID: 19307212
- PMCID: PMC2687359
- DOI: 10.1128/IAI.00166-09
Cytolethal distending toxin promotes Helicobacter cinaedi-associated typhlocolitis in interleukin-10-deficient mice
Abstract
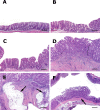
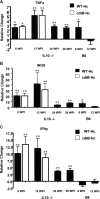
Helicobacter cinaedi colonizes a wide host range, including rodents, and may be an emerging zoonotic agent. Colonization parameters, pathology, serology, and inflammatory responses to wild-type H. cinaedi (WT(Hc)) were evaluated in B6.129P2-IL-10(tm1Cgn) (IL-10(-/-)) mice for 36 weeks postinfection (WPI) and in C57BL/6 (B6) mice for 12 WPI. Because cytolethal distending toxin (CDT) may be a virulence factor, IL-10(-/-) mice were also infected with the cdtB(Hc) and cdtB-N(Hc) isogenic mutants and evaluated for 12 WPI. Consistent with other murine enterohepatic helicobacters, WT(Hc) did not cause typhlocolitis in B6 mice, but mild to severe lesions developed at the cecocolic junction in IL-10(-/-) mice, despite similar colonization levels of WT(Hc) in the cecum and colon of both B6 and IL-10(-/-) mice. WT(Hc) and cdtB mutants also colonized IL-10(-/-) mice to a similar extent, but infection with either cdtB mutant resulted in attenuated typhlocolitis and hyperplasia compared to infection with WT(Hc) (P < 0.03), and only WT(Hc) infection caused dysplasia and intramucosal carcinoma. WT(Hc) and cdtB(Hc) mutant infection of IL-10(-/-) mice elevated mRNA expression of tumor necrosis factor alpha, inducible nitric oxide synthase, and gamma interferon in the cecum, as well as elevated Th1-associated serum immunoglobulin G2a(b) compared to infection of B6 mice (P < 0.05). Although no hepatitis was noted, liver samples were PCR positive at various time points for WT(Hc) or the cdtB(Hc) mutant in approximately 33% of IL-10(-/-) mice and in 10 to 20% of WT(Hc)-infected B6 mice. These results indicate that WT(Hc) can be used to model inflammatory bowel disease in IL-10(-/-) mice and that CDT contributes to the virulence of H. cinaedi.
Figures






References
-
- Boivin, G. P., K. Washington, and K. Yang. 2003. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastoenterology 124762-777. - PubMed
-
- Burman, W. J., D. L. Cohn, R. R. Reves, and M. L. Wilson. 1995. Multifocal cellulitis and monoarticular arthritis as manifestations of Helicobacter cinaedi bacteremia. Clin. Infect. Dis. 20564-570. - PubMed
-
- Chien, C. C., N. S. Taylor, Z. Ge, D. B. Schauer, V. B. Young, and J. G. Fox. 2000. Identification of cdtB homologues and cytolethal distending toxin activity in enterohepatic Helicobacter spp. J. Med. Microbiol. 49525-534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

