Cdk2 and Cdk4 activities are dispensable for tumorigenesis caused by the loss of p53
- PMID: 19307310
- PMCID: PMC2682046
- DOI: 10.1128/MCB.00952-08
Cdk2 and Cdk4 activities are dispensable for tumorigenesis caused by the loss of p53
Abstract
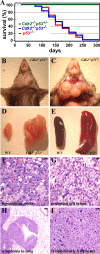
The loss of p53 induces spontaneous tumors in mice, and p53 mutations are found in approximately 50% of human tumors. These tumors are generally caused by a number of events, including genomic instability, checkpoint defects, mitotic defects, deregulation of transcriptional targets, impaired apoptosis, and G(1) deregulation or a combination of these effects. In order to determine the role of proteins involved in G(1) control in tumorigenesis, we focused on Cdk2 and Cdk4, two cyclin-dependent kinases that in association with cyclin E and cyclin D promote the G(1)/S phase transition. We analyzed the consequence of loss of Cdk2 in p53-null animals by generating Cdk2(-/-) p53(-/-) mice. These mice are viable and developed spontaneous tumors, predominantly lymphoblastic lymphomas, similar to p53(-/-) mice. In contrast, the genotypes Cdk4(-/-) p53(-/-) were mostly lethal, with few exceptions, and Cdk2(-/-) Cdk4(-/-) p53(-/-) mice die during embryogenesis at embryonic day 13.5. To study the oncogenic potential, we generated mouse embryonic fibroblasts (MEFs) and found that p53(-/-), Cdk2(-/-) p53(-/-), Cdk4(-/-) p53(-/-), and Cdk2(-/-) Cdk4(-/-) p53(-/-) MEFs grew at similar rates without entering senescence. Ras-transformed MEFs of these genotypes were able to form colonies in vitro and induce tumors in nude mice. Our results suggest that tumorigenicity mediated by p53 loss does not require either Cdk2 or Cdk4, which necessitates considering the use of broad-spectrum cell cycle inhibitors as a means of effective anti-Cdk cancer therapy.
Figures







Similar articles
-
Cdk2 and Cdk4 regulate the centrosome cycle and are critical mediators of centrosome amplification in p53-null cells.Mol Cell Biol. 2010 Feb;30(3):694-710. doi: 10.1128/MCB.00253-09. Epub 2009 Nov 23. Mol Cell Biol. 2010. PMID: 19933848 Free PMC article.
-
Mice thrive without Cdk4 and Cdk2.Mol Oncol. 2007 Jun;1(1):72-83. doi: 10.1016/j.molonc.2007.03.001. Epub 2007 Mar 14. Mol Oncol. 2007. PMID: 19383288 Free PMC article.
-
Requirements for p53 and the ATM gene product in the regulation of G1/S and S phase checkpoints.Oncogene. 1998 Feb 12;16(6):721-36. doi: 10.1038/sj.onc.1201793. Oncogene. 1998. PMID: 9488036
-
A confederacy of kinases: Cdk2 and Cdk4 conspire to control embryonic cell proliferation.Mol Cell. 2006 May 19;22(4):432-3. doi: 10.1016/j.molcel.2006.05.006. Mol Cell. 2006. PMID: 16713571 Review.
-
[Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology].Cancer Radiother. 2001 Apr;5(2):109-29. doi: 10.1016/s1278-3218(01)00087-7. Cancer Radiother. 2001. PMID: 11355576 Review. French.
Cited by
-
MDM2 antagonists overcome intrinsic resistance to CDK4/6 inhibition by inducing p21.Sci Transl Med. 2019 Aug 14;11(505):eaav7171. doi: 10.1126/scitranslmed.aav7171. Sci Transl Med. 2019. PMID: 31413145 Free PMC article.
-
Cyclin-dependent kinase 1 (Cdk1) is essential for cell division and suppression of DNA re-replication but not for liver regeneration.Proc Natl Acad Sci U S A. 2012 Mar 6;109(10):3826-31. doi: 10.1073/pnas.1115201109. Epub 2012 Feb 21. Proc Natl Acad Sci U S A. 2012. PMID: 22355113 Free PMC article.
-
Combined Inactivation of Pocket Proteins and APC/CCdh1 by Cdk4/6 Controls Recovery from DNA Damage in G1 Phase.Cells. 2021 Mar 4;10(3):550. doi: 10.3390/cells10030550. Cells. 2021. PMID: 33806417 Free PMC article.
-
Predicting phase-I metabolism of piceatannol: an in silico study.In Silico Pharmacol. 2024 Jun 5;12(1):52. doi: 10.1007/s40203-024-00228-x. eCollection 2024. In Silico Pharmacol. 2024. PMID: 38854674 Free PMC article.
-
In vitro growth inhibition of human cancer cells by novel honokiol analogs.Bioorg Med Chem. 2012 May 15;20(10):3202-11. doi: 10.1016/j.bmc.2012.03.062. Epub 2012 Apr 3. Bioorg Med Chem. 2012. PMID: 22533983 Free PMC article.
References
-
- Ababneh, M., C. Gotz, and M. Montenarh. 2001. Downregulation of the Cdc2/cyclin B protein kinase activity by binding of p53 to p34Cdc2. Biochem. Biophys. Res. Commun. 283507-512. - PubMed
-
- Aleem, E., H. Kiyokawa, and P. Kaldis. 2005. Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat. Cell Biol. 7831-836. - PubMed
-
- Berthet, C., E. Aleem, V. Coppola, L. Tessarollo, and P. Kaldis. 2003. Cdk2 knockout mice are viable. Curr. Biol. 131775-1785. - PubMed
-
- Berthet, C., K. D. Klarmann, M. B. Hilton, H. C. Suh, J. R. Keller, H. Kiyokawa, and P. Kaldis. 2006. Combined loss of Cdk2 and Cdk4 results in embryonic lethality and Rb hypophosphorylation. Dev. Cell 10563-573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
