Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease
- PMID: 19307473
- PMCID: PMC2717745
- DOI: 10.1161/CIRCULATIONAHA.108.827972
Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease
Abstract
Background: Clinical studies have demonstrated that 50% of individuals with chronic renal disease (CRD) die of cardiovascular causes, including advanced calcific arterial and valvular disease; however, the mechanisms of accelerated calcification in CRD remain obscure, and no therapies can prevent disease progression. We recently demonstrated in vivo that inflammation triggers cardiovascular calcification. In vitro evidence also indicates that elastin degradation products may promote osteogenesis. Here, we used genetically modified mice and molecular imaging to test the hypothesis in vivo that cathepsin S (catS), a potent elastolytic proteinase, accelerates calcification in atherosclerotic mice with CRD induced by 5/6 nephrectomy.
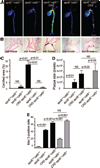
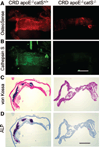
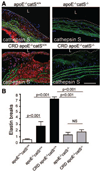
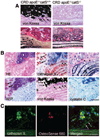
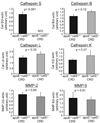
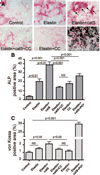
Methods and results: Apolipoprotein-deficient (apoE(-/-))/catS(+/+) (n=24) and apoE(-/-)/catS(-/-) (n=24) mice were assigned to CRD and control groups. CRD mice had significantly higher serum phosphate, creatinine, and cystatin C levels than those without CRD. To visualize catS activity and osteogenesis in vivo, we coadministered catS-activatable and calcification-targeted molecular imaging agents 10 weeks after nephrectomy. Imaging coregistered increased catS and osteogenic activities in the CRD apoE(-/-)/catS(+/+) cohort, whereas CRD apoE(-/-)/catS(-/-) mice exhibited less calcification. Quantitative histology demonstrated greater catS-associated elastin fragmentation and calcification in CRD apoE(-/-)/catS(+/+) than CRD apoE(-/-)/catS(-/-) aortas and aortic valves. Notably, catS deletion did not cause compensatory increases in RNA levels of other elastolytic cathepsins or matrix metalloproteinases. Elastin peptide and recombinant catS significantly increased calcification in smooth muscle cells in vitro, a process further amplified in phosphate-enriched culture medium.
Conclusions: The present study provides direct in vivo evidence that catS-induced elastolysis accelerates arterial and aortic valve calcification in CRD, providing new insight into the pathophysiology of cardiovascular calcification.
Figures


References
-
- Otto CM. Calcific aortic stenosis: time to look more closely at the valve. N Engl J Med. 2008;359:1395–1398. - PubMed
-
- Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. - PubMed
-
- Towler DA. Vascular calcification: a perspective on an imminent disease epidemic. IBMS BoneKEy. 2008;5:41–58.
-
- Fox CS, Larson MG, Vasan RS, Guo CY, Parise H, Levy D, Leip EP, O’Donnell CJ, D’Agostino RB, Sr, Benjamin EJ. Cross-sectional association of kidney function with valvular and annular calcification: the Framingham Heart Study. J Am Soc Nephrol. 2006;17:521–527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

