Phase II studies of gemcitabine and cisplatin in heavily and minimally pretreated metastatic breast cancer
- PMID: 19307510
- PMCID: PMC2674003
- DOI: 10.1200/JCO.2008.17.4839
Phase II studies of gemcitabine and cisplatin in heavily and minimally pretreated metastatic breast cancer
Abstract
Purpose: Cisplatin and gemcitabine have single-agent activity in metastatic breast cancer, and preclinical data support synergy of the combination. Two parallel, phase II trials were conducted to evaluate the response rate, response duration, and toxicities of the combination. Genetic polymorphisms were analyzed for correlation with outcomes.
Patients and methods: Eligible women had measurable disease and heavily or minimally pretreated metastatic breast cancer. The heavily pretreated protocol required prior anthracycline and taxane therapy; cisplatin as part of high-dose therapy was allowed. All patients received cisplatin 25 mg/m(2) on days 1 through 4 and gemcitabine 1,000 mg/m(2) on days 2 and 8 of a 21-day cycle with prophylactic granulocyte colony-stimulating factor in the heavily pretreated group. Sera from a subset of patients were evaluated by polymerase chain reaction restriction fragment length polymorphism for polymorphisms in 10 genes of interest.
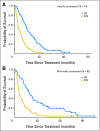
Results: Of 136 women enrolled, 74 were heavily pretreated. Both protocols accrued to their two-stage design. The response rate for both the heavily and minimally pretreated cohorts was 26%, and the median durations of response were 5.3 and 5.9 months, respectively. In a multivariate analysis, hormone receptor-negative disease was associated with a higher response rate. The most common grades 3 or 4 toxicities were thrombocytopenia (71%), neutropenia (66%), and anemia (38%). In a subset of 55 patients, the xeroderma pigmentosum group D (XPD)-751, x-ray cross-complementing group 3 (XRCC3) and cytidine deaminase polymorphisms were significantly associated with clinical outcomes.
Conclusion: Combination cisplatin and gemcitabine is active in metastatic breast cancer regardless of prior therapy. Genetic polymorphisms may tailor which patients benefit from this regimen.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Similar articles
-
Low dose gemcitabine plus cisplatin in a weekly-based regimen as salvage therapy for relapsed breast cancer after taxane-anthracycline-containing regimens.Clin Transl Oncol. 2007 Jul;9(7):459-64. doi: 10.1007/s12094-007-0085-5. Clin Transl Oncol. 2007. PMID: 17652060 Clinical Trial.
-
Gemcitabine and cisplatin combination regimen in patients with anthracycline- and taxane-pretreated metastatic breast cancer.Med Oncol. 2012 Mar;29(1):56-61. doi: 10.1007/s12032-010-9814-7. Epub 2011 Jan 25. Med Oncol. 2012. PMID: 21264537 Clinical Trial.
-
Biweekly administration of gemcitabine and cisplatin chemotherapy in patients with anthracycline and taxane-pretreated metastatic breast cancer.Invest New Drugs. 2008 Aug;26(4):363-8. doi: 10.1007/s10637-007-9110-3. Epub 2007 Dec 29. Invest New Drugs. 2008. PMID: 18165864 Clinical Trial.
-
Gemcitabine combination chemotherapy in metastatic breast cancer: phase II experience.Oncology (Williston Park). 2003 Dec;17(12 Suppl 14):15-21. Oncology (Williston Park). 2003. PMID: 14768400 Review.
-
Gemcitabine plus cisplatin for the treatment of metastatic breast cancer.Clin Breast Cancer. 2002 May;3 Suppl 1:24-9. doi: 10.3816/cbc.2002.s.006. Clin Breast Cancer. 2002. PMID: 12057042 Review.
Cited by
-
Quantification of chemotherapeutic target gene mRNA expression in human breast cancer biopsies: comparison of real-time reverse transcription-PCR vs. relative quantification reverse transcription-PCR utilizing DNA sequencer analysis of PCR products.J Clin Lab Anal. 2003;17(5):184-94. doi: 10.1002/jcla.10091. J Clin Lab Anal. 2003. PMID: 12938148 Free PMC article. Clinical Trial.
-
Genetic polymorphisms and response to 5-fluorouracil, doxorubicin and cyclophosphamide chemotherapy in breast cancer patients.Oncotarget. 2016 Oct 11;7(41):66790-66808. doi: 10.18632/oncotarget.11053. Oncotarget. 2016. PMID: 27527855 Free PMC article.
-
The triterpenoid cucurbitacin B augments the antiproliferative activity of chemotherapy in human breast cancer.Int J Cancer. 2013 Jun 15;132(12):2730-7. doi: 10.1002/ijc.27950. Epub 2012 Dec 13. Int J Cancer. 2013. PMID: 23165325 Free PMC article.
-
Efficacy and safety of gemcitabine plus S-1 vs. gemcitabine plus nab-paclitaxel in treatment-naïve advanced pancreatic ductal adenocarcinoma.Cancer Biol Med. 2023 Aug 29;20(10):765-78. doi: 10.20892/j.issn.2095-3941.2023.0189. Cancer Biol Med. 2023. PMID: 37646237 Free PMC article.
-
Association of Tartrate-Resistant Acid Phosphatase-Expressed Macrophages and Metastatic Breast Cancer Progression.Medicine (Baltimore). 2015 Dec;94(48):e2165. doi: 10.1097/MD.0000000000002165. Medicine (Baltimore). 2015. PMID: 26632898 Free PMC article.
References
-
- Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. - PubMed
-
- Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: Results from NSABP B-28. J Clin Oncol. 2005;23:3686–3696. - PubMed
-
- Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313. - PubMed
-
- Sledge GW, Jr, Loehrer PJ, Sr, Roth BJ, et al. Cisplatin as first-line therapy for metastatic breast cancer. J Clin Oncol. 1988;6:1811–1814. - PubMed
-
- Carmichael J, Possinger K, Phillip P, et al. Advanced breast cancer: A phase II trial with gemcitabine. J Clin Oncol. 1995;13:2731–2736. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

