Mammalian cell penetration, siRNA transfection, and DNA transfection by supercharged proteins
- PMID: 19307578
- PMCID: PMC2659711
- DOI: 10.1073/pnas.0807883106
Mammalian cell penetration, siRNA transfection, and DNA transfection by supercharged proteins
Abstract
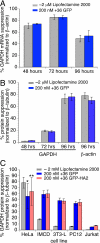
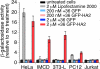
Nucleic acid reagents, including small interfering RNA (siRNA) and plasmid DNA, are important tools for the study of mammalian cells and are promising starting points for the development of new therapeutic agents. Realizing their full potential, however, requires nucleic acid delivery reagents that are simple to prepare, effective across many mammalian cell lines, and nontoxic. We recently described the extensive surface mutagenesis of proteins in a manner that dramatically increases their net charge. Here, we report that superpositively charged green fluorescent proteins, including a variant with a theoretical net charge of +36 (+36 GFP), can penetrate a variety of mammalian cell lines. Internalization of +36 GFP depends on nonspecific electrostatic interactions with sulfated proteoglycans present on the surface of most mammalian cells. When +36 GFP is mixed with siRNA, protein-siRNA complexes approximately 1.7 mum in diameter are formed. Addition of these complexes to five mammalian cell lines, including four that are resistant to cationic lipid-mediated siRNA transfection, results in potent siRNA delivery. In four of these five cell lines, siRNA transfected by +36 GFP suppresses target gene expression. We show that +36 GFP is resistant to proteolysis, is stable in the presence of serum, and extends the serum half-life of siRNA and plasmid DNA with which it is complexed. A variant of +36 GFP can mediate DNA transfection, enabling plasmid-based gene expression. These findings indicate that superpositively charged proteins can overcome some of the key limitations of currently used transfection agents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Engineering and identifying supercharged proteins for macromolecule delivery into mammalian cells.Methods Enzymol. 2012;503:293-319. doi: 10.1016/B978-0-12-396962-0.00012-4. Methods Enzymol. 2012. PMID: 22230574 Free PMC article.
-
Lipoplexes versus nanoparticles: pDNA/siRNA delivery.Drug Deliv. 2013 Feb;20(2):57-64. doi: 10.3109/10717544.2012.752419. Drug Deliv. 2013. PMID: 23537464
-
Structure-transfection activity relationships in a series of novel cationic lipids with heterocyclic head-groups.Org Biomol Chem. 2013 Nov 7;11(41):7164-78. doi: 10.1039/c3ob40442k. Org Biomol Chem. 2013. PMID: 24057052
-
Chemically programmed polymers for targeted DNA and siRNA transfection.Top Curr Chem. 2010;296:227-49. doi: 10.1007/128_2010_69. Top Curr Chem. 2010. PMID: 21504104 Review.
-
Single cell optical transfection.J R Soc Interface. 2010 Jun 6;7(47):863-71. doi: 10.1098/rsif.2009.0463. Epub 2010 Jan 11. J R Soc Interface. 2010. PMID: 20064901 Free PMC article. Review.
Cited by
-
Functional peptides for siRNA delivery.Adv Drug Deliv Rev. 2017 Feb;110-111:157-168. doi: 10.1016/j.addr.2016.08.004. Epub 2016 Aug 13. Adv Drug Deliv Rev. 2017. PMID: 27530388 Free PMC article. Review.
-
Genetic and Covalent Protein Modification Strategies to Facilitate Intracellular Delivery.Biomacromolecules. 2021 Dec 13;22(12):4883-4904. doi: 10.1021/acs.biomac.1c00745. Epub 2021 Dec 2. Biomacromolecules. 2021. PMID: 34855385 Free PMC article. Review.
-
Artificial cell membrane binding thrombin constructs drive in situ fibrin hydrogel formation.Nat Commun. 2019 Apr 23;10(1):1887. doi: 10.1038/s41467-019-09763-0. Nat Commun. 2019. PMID: 31015421 Free PMC article.
-
Enhanced immunogenicity of a positively supercharged archaeon thioredoxin scaffold as a cell-penetrating antigen carrier for peptide vaccines.Front Immunol. 2022 Aug 9;13:958123. doi: 10.3389/fimmu.2022.958123. eCollection 2022. Front Immunol. 2022. PMID: 36032169 Free PMC article.
-
Potent delivery of functional proteins into Mammalian cells in vitro and in vivo using a supercharged protein.ACS Chem Biol. 2010 Aug 20;5(8):747-52. doi: 10.1021/cb1001153. ACS Chem Biol. 2010. PMID: 20545362 Free PMC article.
References
-
- Carlotti F, et al. Lentiviral vectors efficiently transduce quiescent mature 3T3–L1 adipocytes. Mol Ther. 2004;9:209–217. - PubMed
-
- Ma H, et al. Non-classical nuclear localization signal peptides for high efficiency lipofection of primary neurons and neuronal cell lines. Neuroscience. 2002;112:1–5. - PubMed
-
- McManus MT, et al. Small interfering RNA-mediated gene silencing in T lymphocytes. J Immunol. 2002;169:5754–5760. - PubMed
-
- Strait KA, Stricklett PK, Kohan JL, Miller MB, Kohan DE. Calcium regulation of endothelin-1 synthesis in rat inner medullary collecting duct. Am J Physiol. 2007;293:F601–F606. - PubMed
-
- Jantsch J, et al. Small interfering RNA (siRNA) delivery into murine bone marrow-derived dendritic cells by electroporation. J Immunol Methods. 2008;337:71–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

