A family of thermostable fungal cellulases created by structure-guided recombination
- PMID: 19307582
- PMCID: PMC2667002
- DOI: 10.1073/pnas.0901417106
A family of thermostable fungal cellulases created by structure-guided recombination
Abstract
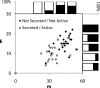
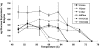
SCHEMA structure-guided recombination of 3 fungal class II cellobiohydrolases (CBH II cellulases) has yielded a collection of highly thermostable CBH II chimeras. Twenty-three of 48 genes sampled from the 6,561 possible chimeric sequences were secreted by the Saccharomyces cerevisiae heterologous host in catalytically active form. Five of these chimeras have half-lives of thermal inactivation at 63 degrees C that are greater than the most stable parent, CBH II enzyme from the thermophilic fungus Humicola insolens, which suggests that this chimera collection contains hundreds of highly stable cellulases. Twenty-five new sequences were designed based on mathematical modeling of the thermostabilities for the first set of chimeras. Ten of these sequences were expressed in active form; all 10 retained more activity than H. insolens CBH II after incubation at 63 degrees C. The total of 15 validated thermostable CBH II enzymes have high sequence diversity, differing from their closest natural homologs at up to 63 amino acid positions. Selected purified thermostable chimeras hydrolyzed phosphoric acid swollen cellulose at temperatures 7 to 15 degrees C higher than the parent enzymes. These chimeras also hydrolyzed as much or more cellulose than the parent CBH II enzymes in long-time cellulose hydrolysis assays and had pH/activity profiles as broad, or broader than, the parent enzymes. Generating this group of diverse, thermostable fungal CBH II chimeras is the first step in building an inventory of stable cellulases from which optimized enzyme mixtures for biomass conversion can be formulated.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
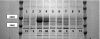


References
-
- Viikari L, Alapuranen M, Puranen T, Vehmaanpera J, Siika-aho M. Thermostable enzymes in lignocellulose hydrolysis. Adv Biocheml Eng Biotechnol. 2007;108:121–145. - PubMed
-
- Szijártó N, et al. Hydrolysis of amorphous and crystalline cellulose by heterologously produced cellulases of Melanocarpus albomyces. J Biotechnol. 2008;156:140–147. - PubMed
-
- Li Y, et al. A diverse family of thermostable cytochrome P450s created by recombination of stabilizing fragments. Nat Biotechnol. 2007;25:1051–1056. - PubMed
-
- Meyer MM, Hochrein L, Arnold FH. Structure-guided SCHEMA recombination of distantly related beta-lactamases. Protein Eng Des Sel. 2006;19:563–570. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

