Nanotechnology approach for drug addiction therapy: gene silencing using delivery of gold nanorod-siRNA nanoplex in dopaminergic neurons
- PMID: 19307583
- PMCID: PMC2667003
- DOI: 10.1073/pnas.0901715106
Nanotechnology approach for drug addiction therapy: gene silencing using delivery of gold nanorod-siRNA nanoplex in dopaminergic neurons
Abstract
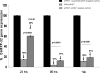
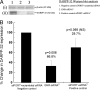
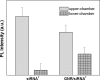
Drug abuse is a worldwide health concern in which addiction involves activation of the dopaminergic signaling pathway in the brain. Here, we introduce a nanotechnology approach that utilizes gold nanorod-DARPP-32 siRNA complexes (nanoplexes) that target this dopaminergic signaling pathway in the brain. The shift in the localized longitudinal plasmon resonance peak of gold nanorods (GNRs) was used to show their interaction with siRNA. Plasmonic enhanced dark field imaging was used to visualize the uptake of these nanoplexes in dopaminergic neurons in vitro. Gene silencing of the nanoplexes in these cells was evidenced by the reduction in the expression of key proteins (DARPP-32, ERK, and PP-1) belonging to this pathway, with no observed cytotoxicity. Moreover, these nanoplexes were shown to transmigrate across an in vitro model of the blood-brain barrier (BBB). Therefore, these nanoplexes appear to be suited for brain-specific delivery of appropriate siRNA for therapy of drug addiction and other brain diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Prasad PN. Introduction to Biophotonics. New York: Wiley; 2004.
-
- Kang H, DeLong R, Fisher MH, Juliano R. Tat-conjugated PAMAM dendrimers as delivey agents for antisense and siRNA oligonucleotides. J Pharm Res. 2005;22(12):2099–2106. - PubMed
-
- Farokhzad OC, Langer R. Nanomedicine: Developing smarter therapeutic and diagnostic modalities. Adv Drug Delivery Rev. 2006;58(14):1456–1459. - PubMed
-
- Pardridge WM. Re-engineering biopharmaceuticals for delivery to brain with molecular Trojan horses. Bioconjug Chem. 2008;19(7):1327–1338. - PubMed
-
- Ferrari M. Cancer nanotechnology: Opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

