N-glycans are direct determinants of CFTR folding and stability in secretory and endocytic membrane traffic
- PMID: 19307599
- PMCID: PMC2699153
- DOI: 10.1083/jcb.200808124
N-glycans are direct determinants of CFTR folding and stability in secretory and endocytic membrane traffic
Abstract
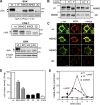
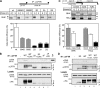
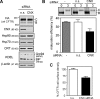
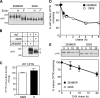
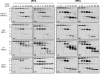
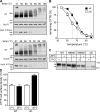
N-glycosylation, a common cotranslational modification, is thought to be critical for plasma membrane expression of glycoproteins by enhancing protein folding, trafficking, and stability through targeting them to the ER folding cycles via lectin-like chaperones. In this study, we show that N-glycans, specifically core glycans, enhance the productive folding and conformational stability of a polytopic membrane protein, the cystic fibrosis transmembrane conductance regulator (CFTR), independently of lectin-like chaperones. Defective N-glycosylation reduces cell surface expression by impairing both early secretory and endocytic traffic of CFTR. Conformational destabilization of the glycan-deficient CFTR induces ubiquitination, leading to rapid elimination from the cell surface. Ubiquitinated CFTR is directed to lysosomal degradation instead of endocytic recycling in early endosomes mediated by ubiquitin-binding endosomal sorting complex required for transport (ESCRT) adaptors Hrs (hepatocyte growth factor-regulated tyrosine kinase substrate) and TSG101. These results suggest that cotranslational N-glycosylation can exert a chaperone-independent profolding change in the energetic of CFTR in vivo as well as outline a paradigm for the peripheral trafficking defect of membrane proteins with impaired glycosylation.
Figures



References
-
- Agashe V.R., Guha S., Chang H.C., Genevaux P., Hayer-Hartl M., Stemp M., Georgopoulos C., Hartl F.U., Barral J.M. 2004. Function of trigger factor and DnaK in multidomain protein folding: increase in yield at the expense of folding speed.Cell. 117:199–209 - PubMed
-
- Arvan P., Zhao X., Ramos-Castaneda J., Chang A. 2002. Secretory pathway quality control operating in Golgi, plasmalemmal, and endosomal systems.Traffic. 3:771–780 - PubMed
-
- Asano T., Takata K., Katagiri H., Ishihara H., Inukai K., Anai M., Hirano H., Yazaki Y., Oka Y. 1993. The role of N-glycosylation in the targeting and stability of GLUT1 glucose transporter.FEBS Lett. 324:258–261 - PubMed
-
- Balch W.E., Morimoto R.I., Dillin A., Kelly J.W. 2008. Adapting proteostasis for disease intervention.Science. 319:916–919 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

