A role for ubiquitin ligases and Spartin/SPG20 in lipid droplet turnover
- PMID: 19307600
- PMCID: PMC2699154
- DOI: 10.1083/jcb.200808041
A role for ubiquitin ligases and Spartin/SPG20 in lipid droplet turnover
Abstract
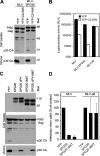
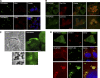
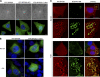
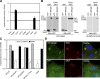
HECT (homologous to the E6AP C terminus) ubiquitin ligases have diverse functions in eukaryotic cells. In screens for proteins that bind to the HECT ubiquitin ligase WWP1, we identified Spartin, which is also known as SPG20. This protein is truncated in a neurological disease, Troyer syndrome. In this study, we show that SPG20 associates with the surface of lipid droplets (LDs) and can regulate their size and number. SPG20 binds to another LD protein, TIP47, and both proteins compete with an additional LD protein, adipophilin/adipocyte differentiation-related protein, for occupancy of LDs. The mutant SPG20 present in Troyer syndrome does not possess these activities. Depletion of SPG20 using RNA interference increases the number and size of LDs when cells are fed with oleic acid. Binding of WWP1 to SPG20 and the consequent ubiquitin transfer remove SPG20 from LDs and reduce the levels of coexpressed SPG20. These experiments suggest functions for ubiquitin ligases and SPG20 in the regulation of LD turnover and potential pathological mechanisms in Troyer syndrome.
Figures

Similar articles
-
Spartin activates atrophin-1-interacting protein 4 (AIP4) E3 ubiquitin ligase and promotes ubiquitination of adipophilin on lipid droplets.BMC Biol. 2010 May 26;8:72. doi: 10.1186/1741-7007-8-72. BMC Biol. 2010. PMID: 20504295 Free PMC article.
-
Spg20-/- mice reveal multimodal functions for Troyer syndrome protein spartin in lipid droplet maintenance, cytokinesis and BMP signaling.Hum Mol Genet. 2012 Aug 15;21(16):3604-18. doi: 10.1093/hmg/dds191. Epub 2012 May 22. Hum Mol Genet. 2012. PMID: 22619377 Free PMC article.
-
S3-12, Adipophilin, and TIP47 package lipid in adipocytes.J Biol Chem. 2005 May 13;280(19):19146-55. doi: 10.1074/jbc.M500978200. Epub 2005 Feb 24. J Biol Chem. 2005. PMID: 15731108
-
PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores.Biochim Biophys Acta. 2009 Jun;1791(6):419-40. doi: 10.1016/j.bbalip.2009.04.002. Epub 2009 Apr 16. Biochim Biophys Acta. 2009. PMID: 19375517 Free PMC article. Review.
-
Fat on the move: intracellular motion of lipid droplets.Biochem Soc Trans. 2009 Oct;37(Pt 5):991-6. doi: 10.1042/BST0370991. Biochem Soc Trans. 2009. PMID: 19754438 Review.
Cited by
-
A C9orf72-CARM1 axis regulates lipid metabolism under glucose starvation-induced nutrient stress.Genes Dev. 2018 Nov 1;32(21-22):1380-1397. doi: 10.1101/gad.315564.118. Epub 2018 Oct 26. Genes Dev. 2018. PMID: 30366907 Free PMC article.
-
A Perspective on the Link between Mitochondria-Associated Membranes (MAMs) and Lipid Droplets Metabolism in Neurodegenerative Diseases.Biology (Basel). 2023 Mar 8;12(3):414. doi: 10.3390/biology12030414. Biology (Basel). 2023. PMID: 36979106 Free PMC article. Review.
-
Spastin binds to lipid droplets and affects lipid metabolism.PLoS Genet. 2015 Apr 13;11(4):e1005149. doi: 10.1371/journal.pgen.1005149. eCollection 2015 Apr. PLoS Genet. 2015. PMID: 25875445 Free PMC article.
-
Spartin-mediated lipid transfer facilitates lipid droplet turnover.Proc Natl Acad Sci U S A. 2024 Jan 16;121(3):e2314093121. doi: 10.1073/pnas.2314093121. Epub 2024 Jan 8. Proc Natl Acad Sci U S A. 2024. PMID: 38190532 Free PMC article.
-
The Many Faces of Lipids in Genome Stability (and How to Unmask Them).Int J Mol Sci. 2021 Nov 29;22(23):12930. doi: 10.3390/ijms222312930. Int J Mol Sci. 2021. PMID: 34884734 Free PMC article. Review.
References
-
- Agarwal A.K., Garg A. 2004. Seipin: a mysterious protein.Trends Mol. Med. 10:440–444 - PubMed
-
- Bakowska J.C., Jenkins R., Pendleton J., Blackstone C. 2005. The Troyer syndrome (SPG20) protein spartin interacts with Eps15.Biochem. Biophys. Res. Commun. 334:1042–1048 - PubMed
-
- Bartz R., Zehmer J.K., Zhu M., Chen Y., Serrero G., Zhao Y., Liu P. 2007. Dynamic activity of lipid droplets: protein phosphorylation and GTP-mediated protein translocation.J. Proteome Res. 6:3256–3265 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

