Absence of proteinase-activated receptor-1 signaling in mice confers protection from fMLP-induced goblet cell metaplasia
- PMID: 19307611
- PMCID: PMC2784406
- DOI: 10.1165/rcmb.2007-0386OC
Absence of proteinase-activated receptor-1 signaling in mice confers protection from fMLP-induced goblet cell metaplasia
Abstract
The morphological features of chronic obstructive pulmonary disease in man include emphysema and chronic bronchitis associated with mucus hypersecretion. These alterations can be induced in mice by a single intratracheal instillation of N-formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP), a chemoattractant and degranulating agent for neutrophils. The mechanisms underlying excessive mucus production and, in particular, goblet cell hyperplasia/metaplasia in chronic obstructive pulmonary disease remain poorly understood. The proteinase-activated receptors (PARs) are widely recognized for their modulatory properties during inflammation. In this study, we examined whether PAR-1 contributes to inflammation and lung damage induced by fMLP by comparing the response of PAR-1-deficient (PAR-1(-/-)) mice with that of wild-type (WT) mice. Mice were killed at various time points after fMLP instillation (200 microg/50 microl). WT mice developed emphysema and goblet cell metaplasia. The onset of pulmonary lesions was preceded by an increase in thrombin immunoreactivity in bronchial airways and alveolar tissue. This was followed by a decrease in PAR-1 immunoreactivity, and by an increase in IL-13 immunostaining on the luminal surface of airway epithelial cells. In PAR-1(-/-) mice, fMLP administration induced similar responses in terms of inflammation and emphysema, but these mice were protected from the development of goblet cell metaplasia. The involvement of PAR-1 in airway epithelial cell transdifferentiation was confirmed by demonstrating that intratracheal instillation of the selective PAR-1 agonist (TFLLR) induced goblet cell metaplasia in the airways of WT mice only. These data suggest that emphysema and goblet cell metaplasia occur independently, and that PAR-1 signaling through IL-13 stimulation may play an important role in inducing goblet cell metaplasia.
Figures

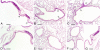


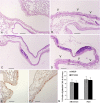


References
-
- Saetta M, Turato G, Lupi F, Fabbri LM. Inflammation in the pathogenesis of chronic obstructive pulmonary disease. In: Voelkel NF and MacNee W, editors. Chronic obstructive lung disease. Hamilton, ON, Canada: BC Decker; 2002. pp. 114–126.
-
- Minematsu N, Shapiro SD. To live and die in the la (lung airway): mode of neutrophil death and progression of chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2007;37:129–130. - PubMed
-
- Tetley TD. Inflammatory cells and chronic obstructive pulmonary disease. Curr Drug Targets Inflamm Allergy 2005;4:607–618. - PubMed
-
- Nadel JA. Mucus and mucus-secreting cells. In: Voelkel NF and MacNee W, editors. Chronic obstructive lung disease. Hamilton, ON, Canada: BC Decker; 2002. pp.161–174.
-
- Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev 2004;56:515–548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

