Loss of serum response factor in keratinocytes results in hyperproliferative skin disease in mice
- PMID: 19307725
- PMCID: PMC2662566
- DOI: 10.1172/JCI37771
Loss of serum response factor in keratinocytes results in hyperproliferative skin disease in mice
Abstract
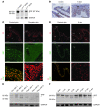
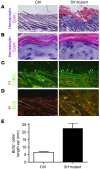
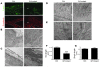
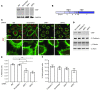
The transcription factor serum response factor (SRF) plays a crucial role in the development of several organs. However, its role in the skin has not been explored. Here, we show that keratinocytes in normal human and mouse skin expressed high levels of SRF but that SRF expression was strongly downregulated in the hyperproliferative epidermis of wounded and psoriatic skin. Keratinocyte-specific deletion within the mouse SRF locus during embryonic development caused edema and skin blistering, and all animals died in utero. Postnatal loss of mouse SRF in keratinocytes resulted in the development of psoriasis-like skin lesions. These lesions were characterized by inflammation, hyperproliferation, and abnormal differentiation of keratinocytes as well as by disruption of the actin cytoskeleton. Ultrastructural analysis revealed markedly reduced cell-cell and cell-matrix contacts and loss of cell compaction in all epidermal layers. siRNA-mediated knockdown of SRF in primary human keratinocytes revealed that the cytoskeletal abnormalities and adhesion defects were a direct consequence of the loss of SRF. In contrast, the hyperproliferation observed in vivo was an indirect effect that was most likely a consequence of the inflammation. These results reveal that loss of SRF disrupts epidermal homeostasis and strongly suggest its involvement in the pathogenesis of hyperproliferative skin diseases, including psoriasis.
Figures



Similar articles
-
Serum response factor controls transcriptional network regulating epidermal function and hair follicle morphogenesis.J Invest Dermatol. 2013 Mar;133(3):608-617. doi: 10.1038/jid.2012.378. Epub 2012 Nov 15. J Invest Dermatol. 2013. PMID: 23151848
-
MiR-217 is down-regulated in psoriasis and promotes keratinocyte differentiation via targeting GRHL2.Biochem Biophys Res Commun. 2016 Feb 26;471(1):169-76. doi: 10.1016/j.bbrc.2016.01.157. Epub 2016 Jan 28. Biochem Biophys Res Commun. 2016. PMID: 26826389
-
A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis.J Clin Invest. 2001 Aug;108(4):527-36. doi: 10.1172/JCI12153. J Clin Invest. 2001. PMID: 11518726 Free PMC article.
-
Epidermal activation of the small GTPase Rac1 in psoriasis pathogenesis.Small GTPases. 2019 May;10(3):163-168. doi: 10.1080/21541248.2016.1273861. Epub 2017 May 4. Small GTPases. 2019. PMID: 28055293 Free PMC article. Review.
-
The Role of Podoplanin in Skin Diseases.Int J Mol Sci. 2022 Jan 24;23(3):1310. doi: 10.3390/ijms23031310. Int J Mol Sci. 2022. PMID: 35163233 Free PMC article. Review.
Cited by
-
Mechanical regulation of chromatin and transcription.Nat Rev Genet. 2022 Oct;23(10):624-643. doi: 10.1038/s41576-022-00493-6. Epub 2022 May 23. Nat Rev Genet. 2022. PMID: 35606569 Review.
-
Desmosomes and Intermediate Filaments: Their Consequences for Tissue Mechanics.Cold Spring Harb Perspect Biol. 2017 Jun 1;9(6):a029157. doi: 10.1101/cshperspect.a029157. Cold Spring Harb Perspect Biol. 2017. PMID: 28096266 Free PMC article. Review.
-
Activation of PPARbeta/delta causes a psoriasis-like skin disease in vivo.PLoS One. 2010 Mar 16;5(3):e9701. doi: 10.1371/journal.pone.0009701. PLoS One. 2010. PMID: 20300524 Free PMC article.
-
The GEF Bcr activates RhoA/MAL signaling to promote keratinocyte differentiation via desmoglein-1.J Cell Biol. 2013 Aug 19;202(4):653-66. doi: 10.1083/jcb.201304133. Epub 2013 Aug 12. J Cell Biol. 2013. PMID: 23940119 Free PMC article.
-
Serum response factor is required for cortical axon growth but is dispensable for neurogenesis and neocortical lamination.J Neurosci. 2011 Nov 16;31(46):16651-64. doi: 10.1523/JNEUROSCI.3015-11.2011. J Neurosci. 2011. PMID: 22090492 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

