Discovery and characterization of novel vascular and hematopoietic genes downstream of etsrp in zebrafish
- PMID: 19308258
- PMCID: PMC2654924
- DOI: 10.1371/journal.pone.0004994
Discovery and characterization of novel vascular and hematopoietic genes downstream of etsrp in zebrafish
Abstract
The transcription factor Etsrp is required for vasculogenesis and primitive myelopoiesis in zebrafish. When ectopically expressed, etsrp is sufficient to induce the expression of many vascular and myeloid genes in zebrafish. The mammalian homolog of etsrp, ER71/Etv2, is also essential for vascular and hematopoietic development. To identify genes downstream of etsrp, gain-of-function experiments were performed for etsrp in zebrafish embryos followed by transcription profile analysis by microarray. Subsequent in vivo expression studies resulted in the identification of fourteen genes with blood and/or vascular expression, six of these being completely novel. Regulation of these genes by etsrp was confirmed by ectopic induction in etsrp overexpressing embryos and decreased expression in etsrp deficient embryos. Additional functional analysis of two newly discovered genes, hapln1b and sh3gl3, demonstrates their importance in embryonic vascular development. The results described here identify a group of genes downstream of etsrp likely to be critical for vascular and/or myeloid development.
Conflict of interest statement
Figures
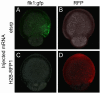




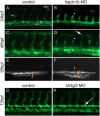
References
-
- Ciau-Uitz A, Walmsley M, Patient R. Distinct origins of adult and embryonic blood in Xenopus. Cell. 2000;102:787–796. - PubMed
-
- Schmidt A, Brixius K, Bloch W. Endothelial precursor cell migration during vasculogenesis. Circ Res. 2007;101:125–136. - PubMed
-
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. - PubMed
-
- Walmsley M, Ciau-Uitz A, Patient R. Adult and embryonic blood and endothelium derive from distinct precursor populations which are differentially programmed by BMP in Xenopus. Development. 2002;129:5683–5695. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

