C5a enhances dysregulated inflammatory and angiogenic responses to malaria in vitro: potential implications for placental malaria
- PMID: 19308263
- PMCID: PMC2655724
- DOI: 10.1371/journal.pone.0004953
C5a enhances dysregulated inflammatory and angiogenic responses to malaria in vitro: potential implications for placental malaria
Abstract
Background: Placental malaria (PM) is a leading cause of maternal and infant mortality. Although the accumulation of parasitized erythrocytes (PEs) and monocytes within the placenta is thought to contribute to the pathophysiology of PM, the molecular mechanisms underlying PM remain unclear. Based on the hypothesis that excessive complement activation may contribute to PM, in particular generation of the potent inflammatory peptide C5a, we investigated the role of C5a in the pathogenesis of PM in vitro and in vivo.
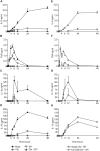
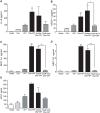
Methodology and principal findings: Using primary human monocytes, the interaction between C5a and malaria in vitro was assessed. CSA- and CD36-binding PEs induced activation of C5 in the presence of human serum. Plasmodium falciparum GPI (pfGPI) enhanced C5a receptor expression (CD88) on monocytes, and the co-incubation of monocytes with C5a and pfGPI resulted in the synergistic induction of cytokines (IL-6, TNF, IL-1beta, and IL-10), chemokines (IL-8, MCP-1, MIP1alpha, MIP1beta) and the anti-angiogenic factor sFlt-1 in a time and dose-dependent manner. This dysregulated response was abrogated by C5a receptor blockade. To assess the potential role of C5a in PM, C5a plasma levels were measured in malaria-exposed primigravid women in western Kenya. Compared to pregnant women without malaria, C5a levels were significantly elevated in women with PM.
Conclusions and significance: These results suggest that C5a may contribute to the pathogenesis of PM by inducing dysregulated inflammatory and angiogenic responses that impair placental function.
Conflict of interest statement
Figures
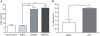
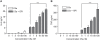

Similar articles
-
Complement activation and the resulting placental vascular insufficiency drives fetal growth restriction associated with placental malaria.Cell Host Microbe. 2013 Feb 13;13(2):215-26. doi: 10.1016/j.chom.2013.01.010. Cell Host Microbe. 2013. PMID: 23414761
-
Lack of an association between antibodies to Plasmodium falciparum glycosylphosphatidylinositols and malaria-associated placental changes in Cameroonian women with preterm and full-term deliveries.Infect Immun. 2004 Sep;72(9):5267-73. doi: 10.1128/IAI.72.9.5267-5273.2004. Infect Immun. 2004. PMID: 15322022 Free PMC article.
-
Myeloperoxidase and Other Markers of Neutrophil Activation Associate With Malaria and Malaria/HIV Coinfection in the Human Placenta.Front Immunol. 2021 Oct 19;12:682668. doi: 10.3389/fimmu.2021.682668. eCollection 2021. Front Immunol. 2021. PMID: 34737733 Free PMC article. Clinical Trial.
-
Molecular mechanisms of Plasmodium falciparum placental adhesion.Cell Microbiol. 2001 Mar;3(3):125-31. doi: 10.1046/j.1462-5822.2001.00109.x. Cell Microbiol. 2001. PMID: 11260135 Review.
-
VAR2CSA-Mediated Host Defense Evasion of Plasmodium falciparum Infected Erythrocytes in Placental Malaria.Front Immunol. 2021 Feb 9;11:624126. doi: 10.3389/fimmu.2020.624126. eCollection 2020. Front Immunol. 2021. PMID: 33633743 Free PMC article. Review.
Cited by
-
Complement Activation in Placental Malaria.Front Microbiol. 2015 Dec 21;6:1460. doi: 10.3389/fmicb.2015.01460. eCollection 2015. Front Microbiol. 2015. PMID: 26733992 Free PMC article.
-
Deletion of carboxypeptidase N delays onset of experimental cerebral malaria.Parasite Immunol. 2012 Aug-Sep;34(8-9):444-7. doi: 10.1111/j.1365-3024.2012.01376.x. Parasite Immunol. 2012. PMID: 22708514 Free PMC article.
-
Dysregulation of angiopoietins is associated with placental malaria and low birth weight.PLoS One. 2010 Mar 1;5(3):e9481. doi: 10.1371/journal.pone.0009481. PLoS One. 2010. PMID: 20208992 Free PMC article.
-
Complement System Activation Is a Plasma Biomarker Signature during Malaria in Pregnancy.Genes (Basel). 2023 Aug 14;14(8):1624. doi: 10.3390/genes14081624. Genes (Basel). 2023. PMID: 37628675 Free PMC article.
-
Molecular Principles of Intrauterine Growth Restriction in Plasmodium Falciparum Infection.Front Endocrinol (Lausanne). 2019 Mar 1;10:98. doi: 10.3389/fendo.2019.00098. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 30930847 Free PMC article. Review.
References
-
- Gilles HM, Lawson JB, Sibelas M, Voller A, Allan N. Malaria, anaemia and pregnancy. Ann Trop Med Parasitol. 1969;63:245–263. - PubMed
-
- Diagne N, Rogier C, Cisse B, Trape JF. Incidence of clinical malaria in pregnant women exposed to intense perennial transmission. Trans R Soc Trop Med Hyg. 1997;91:166–170. - PubMed
-
- Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. - PubMed
-
- Duffy MF, Byrne TJ, Elliott SR, Wilson DW, Rogerson SJ, et al. Broad analysis reveals a consistent pattern of var gene transcription in Plasmodium falciparum repeatedly selected for a defined adhesion phenotype. Mol Microbiol. 2005;56:774–788. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

