MicroRNA-199b-5p impairs cancer stem cells through negative regulation of HES1 in medulloblastoma
- PMID: 19308264
- PMCID: PMC2656623
- DOI: 10.1371/journal.pone.0004998
MicroRNA-199b-5p impairs cancer stem cells through negative regulation of HES1 in medulloblastoma
Abstract
Background: Through negative regulation of gene expression, microRNAs (miRNAs) can function in cancers as oncosuppressors, and they can show altered expression in various tumor types. Here we have investigated medulloblastoma tumors (MBs), which arise from an early impairment of developmental processes in the cerebellum, where Notch signaling is involved in many cell-fate-determining stages. MBs occur bimodally, with the peak incidence seen between 3-4 years and 8-9 years of age, although it can also occur in adults. Notch regulates a subset of the MB cells that have stem-cell-like properties and can promote tumor growth. On the basis of this evidence, we hypothesized that miRNAs targeting the Notch pathway can regulated these phenomena, and can be used in anti-cancer therapies.
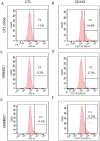
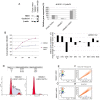
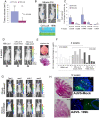
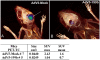
Methodology/principal findings: In a screening of MB cell lines, the miRNA miR-199b-5p was seen to be a regulator of the Notch pathway through its targeting of the transcription factor HES1. Down-regulation of HES1 expression by miR-199b-5p negatively regulates the proliferation rate and anchorage-independent growth of MB cells. MiR-199b-5p over-expression blocks expression of several cancer stem-cell genes, impairs the engrafting potential of MB cells in the cerebellum of athymic/nude mice, and of particular interest, decreases the MB stem-cell-like (CD133+) subpopulation of cells. In our analysis of 61 patients with MB, the expression of miR-199b-5p in the non-metastatic cases was significantly higher than in the metastatic cases (P = 0.001). Correlation with survival for these patients with high levels of miR-199b expression showed a positive trend to better overall survival than for the low-expressing patients. These data showing the down-regulation of miR-199b-5p in metastatic MBs suggest a potential silencing mechanism through epigenetic or genetic alterations. Upon induction of de-methylation using 5-aza-deoxycytidine, lower miR-199b-5p expression was seen in a panel of MB cell lines, supported an epigenetic mechanism of regulation. Furthermore, two cell lines (Med8a and UW228) showed significant up-regulation of miR-199b-5p upon treatment. Infection with MB cells in an induced xenograft model in the mouse cerebellum and the use of an adenovirus carrying miR-199b-5p indicate a clinical benefit through this negative influence of miR-199b-5p on tumor growth and on the subset of MB stem-cell-like cells, providing further proof of concept.
Conclusions/significance: Despite advances in our understanding of the pathogenesis of MB, one-third of these patients remain incurable and current treatments can significantly damage long-term survivors. Here we show that miR-199b-5p expression correlates with metastasis spread, identifying a new molecular marker for a poor-risk class in patients with MB. We further show that in a xenograft model, MB tumor burden can be reduced, indicating the use of miR199b-5p as an adjuvant therapy after surgery, in combination with radiation and chemotherapy, for the improvement of anti-cancer MB therapies and patient quality of life. To date, this is the first report that expression of a miRNA can deplete the tumor stem cells, indicating an interesting therapeutic approach for the targeting of these cells in brain tumors.
Conflict of interest statement
Figures


References
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
-
- Hammond SM. MicroRNAs as oncogenes. Curr Opin Genet Dev. 2006;16:4–9. - PubMed
-
- O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. - PubMed
-
- Ciafre SA, Galardi S, Mangiola A, Ferracin M, Liu CG, et al. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun. 2005;334:1351–1358. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

