Effect of Nitric Oxide on the Oxygen Metabolism and Growth of E. faecalis
- PMID: 19308272
- PMCID: PMC2654474
- DOI: 10.3164/jcbn.08-235
Effect of Nitric Oxide on the Oxygen Metabolism and Growth of E. faecalis
Abstract
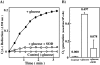
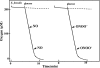
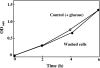
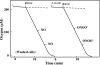
Gastro-intestinal mucosal cells have a potent mechanism to eliminate a variety of pathogens using enzymes that generate reactive oxygen species and/or nitric oxide (NO). However, a large number of bacteria survive in the intestine of human subjects. Enterococcus faecalis (E. faecalis) is a Gram-positive bacterium that survives not only in the intestinal lumen but also within macrophages generating NO. It has been reported that E. faecalis generated the superoxide radical (O(2) (-)). To elucidate the role of O(2) (-) and NO in the mechanism for the pathogen surviving in the intestine and macrophages, we studied the role and metabolism of O(2) (-) and NO in and around E. faecalis. Kinetic analysis revealed that E. faecalis generated 0.5 micromol O(2) (-)/min/10(8) cells in a glucose-dependent manner as determined using the cytochrome c reduction method. The presence of NOC12, an NO donor, strongly inhibited the growth of E. faecalis without affecting in the oxygen consumption. However, the growth rate of NOC12-pretreated E. faecalis in NO-free medium was similar to that of untreated cells. Western blotting analysis revealed that the NOC12-treated E. faecalis revealed a large amount of nitrotyrosine-posititive proteins; the amounts of the modified proteins were higher in cytosol than in membranes. These observations suggested that O(2) (-) generated by E. faecalis reacted with NO to form peroxinitrite (ONOO(-)) that preferentially nitrated tyrosyl residues in cytosolic proteins, thereby reversibly inhibited cellular growth. Since E. faecalis survives even within macrophages expressing NO synthase, similar metabolism of O(2) (-) and NO may occur in and around phagocytized macrophages.
Keywords: Enterococcus faecalis; Superoxide; nitric oxide; nitro-tyrosine; peroxynitrite.
Figures



References
-
- Inoue M., Sato E.F., Nishikawa M., Park A.M., Kira Y., Imada I., Utsumi K. Cross talk of nitric oxide, oxygen radicals, and superoxide dismutase regulates the energy metabolism and cell death and determines the fates of aerobic life. Antioxid. Redox Signal. 2003;5:475–484. - PubMed
-
- Yu H., Sato E.F., Nagata K., Nishikawa M., Kashiba M., Arakawa T., Kobayashi K., Tamura T., Inoue M. Oxygen-dependent regulation of the respiration and growth of Escherichia coli by nitric oxide. FEBS Lett. 1997;409:161–165. - PubMed
-
- Cooper C.E. Nitric oxide and cytochrome oxidase: substrate, inhibitor or effector? Trends Biochem. Sci. 2002;27:33–39. - PubMed

