Vascular smooth muscle cell expression of ectonucleotidase CD39 (ENTPD1) is required for neointimal formation in mice
- PMID: 19308674
- PMCID: PMC2717316
- DOI: 10.1007/s11302-009-9158-y
Vascular smooth muscle cell expression of ectonucleotidase CD39 (ENTPD1) is required for neointimal formation in mice
Abstract
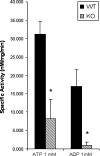
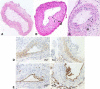
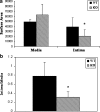
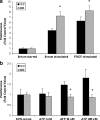
Vascular smooth muscle cell (VSMC) migration and proliferation are critical steps in the pathogenesis of atherosclerosis, post-angioplasty restenosis, neointimal hyperplasia, and chronic allograft rejection. Extracellular nucleotides are known to influence both migration and proliferation of VSMC. Although it is well established that vascular endothelial Cd39/ENTPD1 regulates blood nucleotide concentrations, whether Cd39 associated with VSMC also impacts vascular wall pathology has not been investigated. The objective of this paper is to determine levels of expression of Cd39 on VSMC and functional consequences of gene deletion in vitro and in vivo. Cd39 is the major ectonucleotidase in VSMC, as shown by substantive decreases in ecto-ATPase and -ADPase activity in Cd39-null cells compared to wild type. Significant decreases in neointimal lesion formation are observed in Cd39-null mice at 21 days post arterial balloon injury. Stimulated Cd39-null VSMC have pronounced proliferative responses in vitro. However, using Transwell systems, we show that Cd39-null VSMC fail to migrate in response to ATP, UTP, and PDGF. Cd39 is the dominant ectonucleotidase expressed by VSMC. Deletion of Cd39 in mice results in decreased neointimal formation after vascular injury and is associated with impaired VSMC migration responses in vitro.
Figures

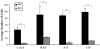
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '8594927', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/8594927/'}]}
- Ellsworth ML, Forrester T, Ellis CG, Dietrich HH (1995) The erythrocyte as a regulator of vascular tone. Am J Physiol 269:H2155–H2161 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1074/jbc.273.11.6334', 'is_inner': False, 'url': 'https://doi.org/10.1074/jbc.273.11.6334'}, {'type': 'PubMed', 'value': '9497362', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9497362/'}]}
- Hamada K, Takuwa N, Yokoyama K, Takuwa Y (1998) Stretch activates Jun N-terminal kinase/stress-activated protein kinase in vascular smooth muscle cells through mechanisms involving autocrine ATP stimulation of purinoceptors. J Biol Chem 273:6334–6340. doi:10.1074/jbc.273.11.6334 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1074/jbc.272.39.24348', 'is_inner': False, 'url': 'https://doi.org/10.1074/jbc.272.39.24348'}, {'type': 'PubMed', 'value': '9305892', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9305892/'}]}
- Lazarowski ER, Homolya L, Boucher RC, Harden TK (1997) Direct demonstration of mechanically induced release of cellular UTP and its implication for uridine nucleotide receptor activation. J Biol Chem 272:24348–24354. doi:10.1074/jbc.272.39.24348 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '2548757', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/2548757/'}]}
- Coad SB, Pearson JD (1989) Metabolism of adenine nucleotides in human blood. Circ Res 65:531–537 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1007/BF01741386', 'is_inner': False, 'url': 'https://doi.org/10.1007/bf01741386'}, {'type': 'PubMed', 'value': '2651791', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/2651791/'}]}
- Luthje J (1997) Origin, metabolism and function of extracellular adenine nucleotides in the blood. Klin Wochenschr 67:317–327. doi:10.1007/BF01741386 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

