Dual FGF-2 and intergrin alpha5beta1 signaling mediate GRAF-induced RhoA inactivation in a model of breast cancer dormancy
- PMID: 19308677
- PMCID: PMC2787927
- DOI: 10.1007/s12307-009-0019-6
Dual FGF-2 and intergrin alpha5beta1 signaling mediate GRAF-induced RhoA inactivation in a model of breast cancer dormancy
Abstract
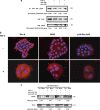
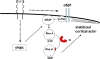
Interactions with the bone marrow stroma regulate dormancy and survival of breast cancer micrometastases. In an in vitro model of dormancy in the bone marrow, we previously demonstrated that estrogen-dependent breast cancer cells are partially re-differentiated by FGF-2, re-express integrin alpha5beta1 lost with malignant transformation and acquire an activated PI3K/Akt pathway. Ligation of integrin alpha5beta1 by fibronectin and activation of the PI3K pathway both contribute to survival of these dormant cells. Here, we investigated mechanisms responsible for the dormant phenotype. Experiments demonstrate that integrin alpha5beta1 controls de novo cytoskeletal rearrangements, cell spreading, focal adhesion kinase rearrangement to the cell perimeter and recruitment of a RhoA GAP known as GRAF. This results in the inactivation of RhoA, an effect which is necessary for the stabilization of cortical actin. Experiments also demonstrate that activation of the PI3K pathway by FGF-2 is independent of integrin alpha5beta1 and is also required for cortical actin reorganization, GRAF membrane relocalization and RhoA inactivation. These data suggest that GRAF-mediated RhoA inactivation and consequent phenotypic changes of dormancy depend on dual signaling by FGF-2-initiated PI3K activation and through ligation of integrin alpha5beta1 by fibronectin.
Figures








References
-
- Braun S, Kentenich C, Janni W, et al. Lack of an effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high risk breast cancer patients. J Clin Onc. 2000;18:80–86. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
