The prometastatic microenvironment of the liver
- PMID: 19308690
- PMCID: PMC2654354
- DOI: 10.1007/s12307-008-0011-6
The prometastatic microenvironment of the liver
Abstract
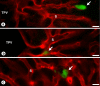
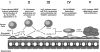
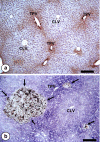
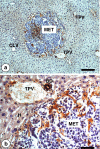
The liver is a major metastasis-susceptible site and majority of patients with hepatic metastasis die from the disease in the absence of efficient treatments. The intrahepatic circulation and microvascular arrest of cancer cells trigger a local inflammatory reaction leading to cancer cell apoptosis and cytotoxicity via oxidative stress mediators (mainly nitric oxide and hydrogen peroxide) and hepatic natural killer cells. However, certain cancer cells that resist or even deactivate these anti-tumoral defense mechanisms still can adhere to endothelial cells of the hepatic microvasculature through proinflammatory cytokine-mediated mechanisms. During their temporary residence, some of these cancer cells ignore growth-inhibitory factors while respond to proliferation-stimulating factors released from tumor-activated hepatocytes and sinusoidal cells. This leads to avascular micrometastasis generation in periportal areas of hepatic lobules. Hepatocytes and myofibroblasts derived from portal tracts and activated hepatic stellate cells are next recruited into some of these avascular micrometastases. These create a private microenvironment that supports their development through the specific release of both proangiogenic factors and cancer cell invasion- and proliferation-stimulating factors. Moreover, both soluble factors from tumor-activated hepatocytes and myofibroblasts also contribute to the regulation of metastatic cancer cell genes. Therefore, the liver offers a prometastatic microenvironment to circulating cancer cells that supports metastasis development. The ability to resist anti-tumor hepatic defense and to take advantage of hepatic cell-derived factors are key phenotypic properties of liver-metastasizing cancer cells. Knowledge on hepatic metastasis regulation by microenvironment opens multiple opportunities for metastasis inhibition at both subclinical and advanced stages. In addition, together with metastasis-related gene profiles revealing the existence of liver metastasis potential in primary tumors, new biomarkers on the prometastatic microenvironment of the liver may be helpful for the individual assessment of hepatic metastasis risk in cancer patients.
Figures




References
-
- None
- Pickren JW, Tsukada Y, Lane WW (1982) Liver metastasis. In: Weiss L, Gilbert HA (eds) Analysis of autopsy data. GK Hall and Company, Boston, Mass, pp 2–18
-
- None
- Gilbert HA, Kagan AR, Hintz BL et al (1982) Patterns of metastases. In: Weiss L, Gilbert HA (eds) Liver metastases. GK Hall Medical Publishers, Boston, MA, pp 19–39
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1038/nrc1098', 'is_inner': False, 'url': 'https://doi.org/10.1038/nrc1098'}, {'type': 'PubMed', 'value': '12778135', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12778135/'}]}
- Fidler IJ (2003) The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3:453–458 - PubMed
-
- None
- Ewing J (1928) Neoplastic diseases, 6th edn. WB Saunders, Philadelphia
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0140-6736(00)49915-0', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0140-6736(00)49915-0'}, {'type': 'PubMed', 'value': '2673568', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/2673568/'}]}
- Paget S (1889) The distribution of secondary growths in cancer of the breast. Lancet 1:571–573 - PubMed
LinkOut - more resources
Full Text Sources
