Microenvironment changes (in pH) affect VEGF alternative splicing
- PMID: 19308691
- PMCID: PMC2654355
- DOI: 10.1007/s12307-008-0013-4
Microenvironment changes (in pH) affect VEGF alternative splicing
Abstract
Vascular endothelial growth factor-A (VEGF-A) has several isoforms, which differ in their capacity to bind extracellular matrix proteins and also in their affinity for VEGF receptors. Although the relative contribution of the VEGF isoforms has been studied in tumor angiogenesis, little is known about the mechanisms that regulate the alternative splicing process. Here, we tested microenvironment cues that might regulate VEGF alternative splicing. To test this, we used endometrial cancer cells that produce all VEGF isoforms as a model, and exposed them to varying pH levels, hormones, glucose and CoCl(2) (to mimic hypoxia). Low pH had the most consistent effects in inducing variations in VEGF splicing pattern (VEGF121 increased significantly, p < 0.001, when compared to VEGF145, 165 or 189). This was accompanied by activation of the p38 stress pathway and SR proteins (splicing factors) expression and phosphorylation. SF2/ASF, SRp20 and SRp40 down-regulation by siRNA impaired the effects of pH stimulation, blocking the shift in VEGF isoforms production. Taken together, we show for the first time that acidosis (low pH) regulates VEGF-A alternative splicing, may be through p38 activation and suggest the possible SR proteins involved in this process.
Figures



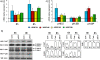
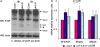


Similar articles
-
Splicing factor polymorphisms, the control of VEGF isoforms and association with angiogenic eye disease.Curr Eye Res. 2011 Apr;36(4):328-35. doi: 10.3109/02713683.2010.548892. Epub 2011 Feb 10. Curr Eye Res. 2011. PMID: 21309690
-
Regulation of SRp20 exon 4 splicing.Biochim Biophys Acta. 2000 Nov 15;1494(1-2):137-43. doi: 10.1016/s0167-4781(00)00233-5. Biochim Biophys Acta. 2000. PMID: 11072076
-
Antagonistic SR proteins regulate alternative splicing of tumor-related Rac1b downstream of the PI3-kinase and Wnt pathways.Hum Mol Genet. 2009 Oct 1;18(19):3696-707. doi: 10.1093/hmg/ddp317. Epub 2009 Jul 13. Hum Mol Genet. 2009. PMID: 19602482
-
Human papillomavirus regulation of SR proteins.Biochem Soc Trans. 2010 Aug;38(4):1116-21. doi: 10.1042/BST0381116. Biochem Soc Trans. 2010. PMID: 20659014 Review.
-
Alternative splicing in angiogenesis: the vascular endothelial growth factor paradigm.Cancer Lett. 2007 May 8;249(2):133-42. doi: 10.1016/j.canlet.2006.08.015. Epub 2006 Oct 5. Cancer Lett. 2007. PMID: 17027147 Review.
Cited by
-
VEGF121 and VEGF165 differentially promote vessel maturation and tumor growth in mice and humans.Cancer Gene Ther. 2016 May;23(5):125-32. doi: 10.1038/cgt.2016.12. Epub 2016 Apr 1. Cancer Gene Ther. 2016. PMID: 27033458
-
The VEGF/VEGFR Axis Revisited: Implications for Cancer Therapy.Int J Mol Sci. 2022 Dec 9;23(24):15585. doi: 10.3390/ijms232415585. Int J Mol Sci. 2022. PMID: 36555234 Free PMC article. Review.
-
Shift in VEGFA isoform balance towards more angiogenic variants is associated with tumor stage and differentiation of human hepatocellular carcinoma.PeerJ. 2018 Jun 5;6:e4915. doi: 10.7717/peerj.4915. eCollection 2018. PeerJ. 2018. PMID: 29888133 Free PMC article.
-
Gene Expressions for Signal Transduction under Acidic Conditions.Genes (Basel). 2013 Mar 8;4(1):65-85. doi: 10.3390/genes4010065. Genes (Basel). 2013. PMID: 24705103 Free PMC article.
-
Engineered culture models for studies of tumor-microenvironment interactions.Annu Rev Biomed Eng. 2013;15:29-53. doi: 10.1146/annurev-bioeng-071811-150028. Epub 2013 Apr 29. Annu Rev Biomed Eng. 2013. PMID: 23642249 Free PMC article. Review.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1111/j.1349-7006.2006.00298.x', 'is_inner': False, 'url': 'https://doi.org/10.1111/j.1349-7006.2006.00298.x'}, {'type': 'PMC', 'value': 'PMC11158478', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC11158478/'}, {'type': 'PubMed', 'value': '16984378', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16984378/'}]}
- Koukourakis MI, Pitiakoudis M, Giatromanolaki A et al (2006) Oxygen and glucose consumption in gastrointestinal adenocarcinomas: correlation with markers of hypoxia, acidity and anaerobic glycolysis. Cancer Sci 97:1056–1060 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1053/j.gastro.2005.06.023', 'is_inner': False, 'url': 'https://doi.org/10.1053/j.gastro.2005.06.023'}, {'type': 'PubMed', 'value': '16344073', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16344073/'}]}
- Sund M, Zeisberg M, Kalluri R (2005) Endogenous stimulators and inhibitors of angiogenesis in gastrointestinal cancers: basic science to clinical application. Gastroenterology 129:2076–2091 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1080/1062332060069110', 'is_inner': False, 'url': 'https://doi.org/10.1080/1062332060069110'}, {'type': 'PubMed', 'value': '16728331', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16728331/'}]}
- Sato Y (2006) Update on endogenous inhibitors of angiogenesis. Endothelium 13:147–155 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1038/nrc1093', 'is_inner': False, 'url': 'https://doi.org/10.1038/nrc1093'}, {'type': 'PubMed', 'value': '12778130', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12778130/'}]}
- Bergers G, Benjamin LE (2003) Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3:401–410 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '1711045', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/1711045/'}]}
- Tischer E, Mitchell R, Hartman T et al (1991) The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem 266:11947–11954 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
