Palladium-catalyzed oxidative intermolecular difunctionalization of terminal alkenes with organostannanes and molecular oxygen
- PMID: 19308939
- PMCID: PMC2844705
- DOI: 10.1002/anie.200900218
Palladium-catalyzed oxidative intermolecular difunctionalization of terminal alkenes with organostannanes and molecular oxygen
Abstract
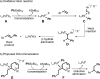
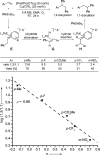
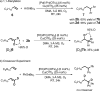
A cationic palladium complex catalyzes the title transformations, which are thought to proceed via a pi-allyl or pi-benzyl intermediate. The regioselectivity of the reaction (1,2- or 1,1-difunctionalization) depends on the type of terminal double bond (conjugated or nonconjugated) in the substrate (see scheme) and appears to be controlled by the relative rates of beta-hydride elimination and transmetalation. DMA=dimethylacetamide, Tf=triflyl.
Figures
References
-
-
For reviews of the Heck reaction, see: Heck RF. Org. React. 1982;27:345–390.; Beletskaya IP, Cheprakov AV. Chem. Rev. 2000;100:3009–3066.; Whitcombe NJ, Hii KK, Gibson SE. Tetrahedron. 2001;57:7449–7476.; Kondolff I, Doucet H, Santelli M. Tetrahedron Lett. 2003;44:8487–8491.
-
-
-
For examples of oxidative Heck reactions, see: Du X, Suguro M, Hirabayashi K, Mori A, Nishikata T, Hagiwara N, Kawata K, Okeda T, Wang HF, Fugami K, Kosugi M. Org. Lett. 2001;3:3313–3316.; Parrish JP, Jung YC, Shin SI, Jung KW. J. Org. Chem. 2002;67:7127–7130.; Jung YC, Mishra RK, Yoon CH, Jung KW. Org. Lett. 2003;5:2231–2234.; Andappan MMS, Nilsson P, von Schenck H, Larhed M. J. Org. Chem. 2004;69:5212–5218.; Yoo KS, Yoon CH, Jung KW. J. Am. Chem. Soc. 2006;128:16384–16393.; Delcamp JH, Brucks AP, White MC. J. Am. Chem. Soc. 2008;130:11270–11271.; for an example in which oxygen and base-free conditions are used, see: Ruan J, Li X, Saidi O, Xiao J. J. Am. Chem. Soc. 2008;130:2424–2425.
-
-
- Organ MG, Chass GA, Fang DC, Hopkinson AC, Valentea C. Synthesis. 2008:2776–2797.
-
-
For an overview of alkene difunctionalization reactions under palladium catalysis, see: Jensen KH, Sigman MS. Org. Biomol. Chem. 2008;6:4083–4088.; for examples of alkene diamination, see: Muniz K, Hovelmann CH, Streuff J. J. Am. Chem. Soc. 2008;130:763–773.; Muniz K, Streuff J, Hovelmann CH, Nunez A. Angew. Chem. 2007;119:7255–7258.; Angew. Chem. Int. Ed. 2007;46:7125–7127.; for an example of aminooxygenation, see: Desai LV, Melanie SS. Angew. Chem. 2007;119:5839–5842.; Angew. Chem. Int. Ed. 2007;46:5737–5740.; for an example of aminoacetoxylation, see: Liu G, Stahl SS. J. Am. Chem. Soc. 2006;128:7179–7181.
-
-
- Kalyani D, Sanford MS. J. Am. Chem. Soc. 2008;130:2150–2151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

