Peptide cyclization and cyclodimerization by Cu(I)-mediated azide-alkyne cycloaddition
- PMID: 19309103
- PMCID: PMC2677176
- DOI: 10.1021/jo802097m
Peptide cyclization and cyclodimerization by Cu(I)-mediated azide-alkyne cycloaddition
Abstract
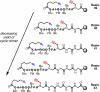
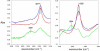
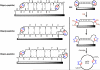
Head-to-tail cyclodimerization of resin-bound oligopeptides bearing azide and alkyne groups occurs readily by 1,3-dipolar cycloaddition upon treatment with Cu(I). The process was found to be independent of peptide sequence, sensitive to the proximity of the alkyne to the resin, sensitive to solvent composition, facile for alpha- and beta-peptides but not for gamma-peptides, and inhibited by the inclusion of tertiary amide linkages. Peptides shorter than hexamers were predominantly converted to cyclic monomers. Oligoglycine and oligo(beta-alanine) chains underwent oligomerization by 1,3-dipolar cycloaddition in the absence of a copper catalyst. These results suggest that cyclodimerization depends on the ability of the azido-alkyne peptide to form in-frame hydrogen bonds between chains in order to place the reacting groups in close proximity and lower the entropic penalty for dimerization. The properties of the resin and solvent are crucial, giving rise to a productive balance between swelling and interstrand H-bonding. These findings allow for the design of optimal substrates for triazole-forming ring closure and for the course of the reaction to be controlled by the choice of conditions.
Figures










References
-
- Davies JS. In: Cyclic Polymers. 2nd Edition Semlyen JA, editor. Kluwer Academic Publishers; Dordrecht, Netherlands: 2000. pp. 85–124.
-
- Davies JS. Amino Acids, Peptides, and Proteins. 2002;33:238–294.
-
- Kessler H, Gratias R, Hessler G, Gurrath M, Mueller G. Pure Appl. Chem. 1996;68:1201–1205.
-
- Colombo G, Curnis F, De Mori GMS, Gasparri A, Longoni C, Sacchi A, Longhi R, Corti A. J. Biol. Chem. 2002;277:47891–47897. - PubMed
-
- Craik DJ, Simonsen S, Daly NL. Curr. Opin. Drug Disc. 2002;5:251–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

