Downstream targets and intracellular compartmentalization in Nox signaling
- PMID: 19309256
- PMCID: PMC2861540
- DOI: 10.1089/ars.2009.2594
Downstream targets and intracellular compartmentalization in Nox signaling
Abstract
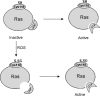
Reactive oxygen species (ROS) have become recognized for their role as second messengers in a multitude of physiologic responses. Emerging evidence points to the importance of the NADPH oxidase family of ROS-producing enzymes in mediating redox-sensitive signal transduction. However, a clear paradox exists between the specificity required for signaling and the nature of ROS as both diffusible and highly reactive molecules. We seek to understand the targets and compartmentalization of the NADPH oxidase signaling to determine how NADPH oxidase-derived ROS fit into established signaling paradigms. Herein we review recent data that link cellular NADPH oxidase enzymes to ROS signaling, with a particular focus on the mechanism(s) involved in achieving signaling specificity.
Figures
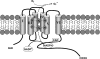




Similar articles
-
Compartmentalization of redox signaling through NADPH oxidase-derived ROS.Antioxid Redox Signal. 2009 Jun;11(6):1289-99. doi: 10.1089/ars.2008.2333. Antioxid Redox Signal. 2009. PMID: 18999986 Free PMC article. Review.
-
ROS signaling and redox biology in endothelial cells.Cell Mol Life Sci. 2015 Sep;72(17):3281-303. doi: 10.1007/s00018-015-1928-9. Epub 2015 May 14. Cell Mol Life Sci. 2015. PMID: 25972278 Free PMC article. Review.
-
Emerging evidence for the importance of phosphorylation in the regulation of NADPH oxidases.Antioxid Redox Signal. 2009 Oct;11(10):2429-41. doi: 10.1089/ars.2009.2590. Antioxid Redox Signal. 2009. PMID: 19358632 Free PMC article. Review.
-
Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets.Diabetes. 2005 Feb;54(2):311-21. doi: 10.2337/diabetes.54.2.311. Diabetes. 2005. PMID: 15677487 Free PMC article. Review.
-
NADPH Oxidases and Measurement of Reactive Oxygen Species.Methods Mol Biol. 2017;1527:219-232. doi: 10.1007/978-1-4939-6625-7_18. Methods Mol Biol. 2017. PMID: 28116720
Cited by
-
Molecular Crosstalk between Integrins and Cadherins: Do Reactive Oxygen Species Set the Talk?J Signal Transduct. 2012;2012:807682. doi: 10.1155/2012/807682. Epub 2011 Dec 13. J Signal Transduct. 2012. PMID: 22203898 Free PMC article.
-
Upregulation of Nox4 induces a pro-survival Nrf2 response in cancer-associated fibroblasts that promotes tumorigenesis and metastasis, in part via Birc5 induction.Breast Cancer Res. 2022 Jul 14;24(1):48. doi: 10.1186/s13058-022-01548-6. Breast Cancer Res. 2022. PMID: 35836253 Free PMC article.
-
Spin biochemistry modulates reactive oxygen species (ROS) production by radio frequency magnetic fields.PLoS One. 2014 Mar 28;9(3):e93065. doi: 10.1371/journal.pone.0093065. eCollection 2014. PLoS One. 2014. PMID: 24681944 Free PMC article.
-
Novel role of p66Shc in ROS-dependent VEGF signaling and angiogenesis in endothelial cells.Am J Physiol Heart Circ Physiol. 2012 Feb 1;302(3):H724-32. doi: 10.1152/ajpheart.00739.2011. Epub 2011 Nov 18. Am J Physiol Heart Circ Physiol. 2012. PMID: 22101521 Free PMC article.
-
Type II diabetes increases mitochondrial DNA mutations in the left ventricle of the Goto-Kakizaki diabetic rat.Am J Physiol Heart Circ Physiol. 2013 Apr 1;304(7):H903-15. doi: 10.1152/ajpheart.00567.2012. Epub 2013 Feb 1. Am J Physiol Heart Circ Physiol. 2013. PMID: 23376826 Free PMC article.
References
-
- Adachi T. Pimentel DR. Heibeck T. Hou X. Lee YJ. Jiang B. Ido Y. Cohen RA. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J Biol Chem. 2004;279:29857–29862. - PubMed
-
- Ago T. Kitazono T. Kuroda J. Kumai Y. Kamouchi M. Ooboshi H. Wakisaka M. Kawahara T. Rokutan K. Ibayashi S. Iida M. NAD(P)H oxidases in rat basilar arterial endothelial cells. Stroke. 2005;36:1040–1046. - PubMed
-
- Banfi B. Clark RA. Steger K. Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. - PubMed
-
- Banfi B. Malgrange B. Knisz J. Steger K. Dubois-Dauphin M. Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

