Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders
- PMID: 19309259
- PMCID: PMC2819800
- DOI: 10.1089/ars.2009.2485
Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders
Abstract
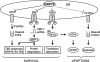
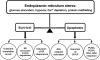
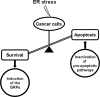
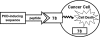
GRP78/BiP is a major endoplasmic reticulum (ER) chaperone protein critical for protein quality control of the ER, as well as controlling the activation of the ER-transmembrane signaling molecules. Through creation of mouse models targeting the Grp78 allele, the function of GRP78 in development and disease has been investigated. These led to the discovery that GRP78 function is obligatory for early embryonic development. However, in adult animals, GRP78 is preferably required for cancer cell survival under pathologic conditions such as tumor progression and drug resistance. The discovery of surface localization of GRP78 in cancer cells reveals potential novel function, interaction with cell-surface receptors, and possible therapeutic implications. Mouse models also reveal that GRP78 controls maturation and secretion of neuronal factors for proper neural migration and offers neuroprotection.
Figures
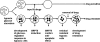
References
-
- Anttonen AK. Mahjneh I. Hamalainen RH. Lagier-Tourenne C. Kopra O. Waris L. Anttonen M. Joensuu T. Kalimo H. Paetau A. Tranebjaerg L. Chaigne D. Koenig M. Eeg-Olofsson O. Udd B. Somer M. Somer H. Lehesjoki AE. The gene disrupted in Marinesco-Sjögren syndrome encodes SIL1, an HSPA5 cochaperone. Nat Genet. 2005;37:1309–1311. - PubMed
-
- Arap MA. Lahdenranta J. Mintz PJ. Hajitou A. Sarkis AS. Arap W. Pasqualini R. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell. 2004;6:275–284. - PubMed
-
- Berger CL. Dong Z. Hanlon D. Bisaccia E. Edelson RL. A ymphocyte cell surface heat shock protein homologous to the endoplasmic reticulum chaperone, immunoglobulin heavy chain binding protein BIP. Int J Cancer. 1997;71:1077–1085. - PubMed
-
- Bhattacharjee G. Ahamed J. Pedersen B. El-Sheikh A. Mackman N. Ruf W. Liu C. Edgington TS. Regulation of tissue factor–mediated initiation of the coagulation cascade by cell surface grp78. Arterioscler Thromb Vasc Biol. 2005;25:1737–1743. - PubMed
-
- Calfon M. Zeng H. Urano F. Till JH. Hubbard SR. Harding HP. Clark SG. Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

