Duodenal intraepithelial lymphocytes of children with cow milk allergy preferentially bind the glycan-binding protein galectin-3
- PMID: 19309568
- PMCID: PMC3844523
- DOI: 10.1177/039463200902200123
Duodenal intraepithelial lymphocytes of children with cow milk allergy preferentially bind the glycan-binding protein galectin-3
Abstract
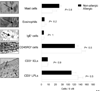
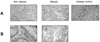
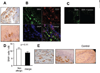
A breakdown in intestinal homeostasis results in inflammatory bowel diseases including coeliac disease and allergy. Galectins, evolutionarily conserved beta-galactoside-binding proteins, can modulate immune-epithelial cell interactions by influencing immune cell fate and cytokine secretion. In this study we investigated the glycosylation signature, as well as the regulated expression of galectin-1 and -3 in human duodenal samples of allergic and non-allergic children. Whereas galectin-1 was predominantly localized in the epithelial compartment (epithelial cells and intraepithelial lymphocytes) and the underlying lamina propria (T cells, macrophages and plasma cells), galectin-3 was mainly expressed by crypt epithelial cells and macrophages in the lamina propria. Remarkably, expression of these galectins was not significantly altered in allergic versus non-allergic patients. Investigation of the glycophenotype of the duodenal inflammatory microenvironment revealed substantial alpha2-6-linked sialic acid bound to galactose in lamina propria plasma cells, macrophages and intraepithelial lymphocytes and significant levels of asialo core 1 O-glycans in CD68+ macrophages and enterocytes. Galectin-1 preferentially bound to neutrophils, plasma cells and enterocytes, while galectin-3 binding sites were mainly distributed on macrophages and intraepithelial lymphocytes. Notably, galectin-3, but not galectin-1 binding, was substantially increased in intraepithelial gut lymphocytes of allergic patients compared to non-allergic subjects, suggesting a potential role of galectin-3-glycan interactions in shaping epithelial-immune cell connections during allergic inflammatory processes.
Figures



Similar articles
-
Defining the glycophenotype of squamous epithelia using plant and mammalian lectins. Differentiation-dependent expression of alpha2,6- and alpha2,3-linked N-acetylneuraminic acid in squamous epithelia and carcinomas, and its differential effect on binding of the endogenous lectins galectins-1 and -3.APMIS. 2002 Dec;110(12):845-56. doi: 10.1034/j.1600-0463.2002.1101202.x. APMIS. 2002. PMID: 12645662
-
Evidence for increased apoptosis of duodenal intraepithelial lymphocytes in cow's milk sensitive enteropathy.J Pediatr Gastroenterol Nutr. 2005 Mar;40(3):352-8. doi: 10.1097/01.mpg.0000151748.07469.bf. J Pediatr Gastroenterol Nutr. 2005. PMID: 15735492
-
Desialylation of airway epithelial cells during influenza virus infection enhances pneumococcal adhesion via galectin binding.Mol Immunol. 2015 May;65(1):1-16. doi: 10.1016/j.molimm.2014.12.010. Epub 2015 Jan 16. Mol Immunol. 2015. PMID: 25597246 Free PMC article.
-
Assembly, organization and regulation of cell-surface receptors by lectin-glycan complexes.Biochem J. 2015 Jul 1;469(1):1-16. doi: 10.1042/BJ20150461. Biochem J. 2015. PMID: 26173257 Review.
-
The immunological potential of galectin-1 and -3.Autoimmun Rev. 2009 Mar;8(5):360-3. doi: 10.1016/j.autrev.2008.11.009. Epub 2008 Dec 6. Autoimmun Rev. 2009. PMID: 19064001 Review.
Cited by
-
The glycan-binding protein galectin-1 controls survival of epithelial cells along the crypt-villus axis of small intestine.Cell Death Dis. 2011 May 26;2(5):e163. doi: 10.1038/cddis.2011.44. Cell Death Dis. 2011. PMID: 21614093 Free PMC article.
-
Microbial Colonization at Early Life Promotes the Development of Diet-Induced CD8αβ Intraepithelial T Cells.Mol Cells. 2019 Apr 30;42(4):313-320. doi: 10.14348/molcells.2019.2431. Mol Cells. 2019. PMID: 30841027 Free PMC article.
-
Type-2 Cytokines Promote the Secretion of the Eosinophil-Attractant CCL26 by Intestinal Epithelial Cells in Food-Sensitized Patients.Front Immunol. 2022 Jun 21;13:909896. doi: 10.3389/fimmu.2022.909896. eCollection 2022. Front Immunol. 2022. PMID: 35799778 Free PMC article.
-
Differential expression of immunomodulatory galectin-1 in peripheral leukocytes and adult tissues and its cytosolic organization in striated muscle.Glycobiology. 2010 May;20(5):507-20. doi: 10.1093/glycob/cwp203. Epub 2010 Jan 5. Glycobiology. 2010. PMID: 20053628 Free PMC article.
-
Development of Galectin-3 Targeting Drugs for Therapeutic Applications in Various Diseases.Int J Mol Sci. 2023 May 1;24(9):8116. doi: 10.3390/ijms24098116. Int J Mol Sci. 2023. PMID: 37175823 Free PMC article. Review.
References
-
- Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2006;117:S470–S475. - PubMed
-
- Romagnani S. The role of lymphocytes in allergic disease. J Allergy Clin Immunol. 2000;105:399–408. - PubMed
-
- Frydas S, Karagouni E, Hatzistilianou M, et al. Cytokines and allergic disorders: revisited study. Int J Immunopathol Pharmacol. 2004;17:233–235. - PubMed
-
- Augustin M, Karttunen TJ, Kokkonen J. TIA1 and mast cell tryptase in food allergy of children: increase of intraepithelial lymphocytes expressing TIA1 associates with allergy. J Pediatr Gastroenterol Nutr. 2001;32:11–18. - PubMed
-
- van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol. 2008;9:593–601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

