Interleukin-1beta enhances nucleotide-induced and alpha-secretase-dependent amyloid precursor protein processing in rat primary cortical neurons via up-regulation of the P2Y(2) receptor
- PMID: 19317852
- PMCID: PMC2710802
- DOI: 10.1111/j.1471-4159.2009.06048.x
Interleukin-1beta enhances nucleotide-induced and alpha-secretase-dependent amyloid precursor protein processing in rat primary cortical neurons via up-regulation of the P2Y(2) receptor
Abstract
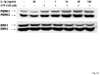
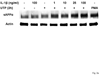
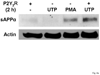
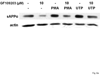
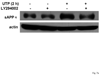
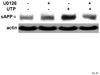
The heterologous expression and activation of the human P2Y(2) nucleotide receptor (P2Y(2)R) in human 1321N1 astrocytoma cells stimulates alpha-secretase-dependent cleavage of the amyloid precursor protein (APP), causing extracellular release of the non-amyloidogenic protein secreted amyloid precursor protein (sAPPalpha). To determine whether a similar response occurs in a neuronal cell, we analyzed whether P2Y(2)R-mediated production of sAPPalpha occurs in rat primary cortical neurons (rPCNs). In rPCNs, P2Y(2)R mRNA and receptor activity were virtually absent in quiescent cells, whereas overnight treatment with the pro-inflammatory cytokine interleukin-1beta (IL-1beta) up-regulated both P2Y(2)R mRNA expression and receptor activity by four-fold. The up-regulation of the P2Y(2)R was abrogated by pre-incubation with Bay 11-7085, an IkappaB-alpha phosphorylation inhibitor, which suggests that P2Y(2)R mRNA transcript levels are regulated through nuclear factor-kappa-B (NFkappaB) signaling. Furthermore, the P2Y(2)R agonist Uridine-5'-triphosphate (UTP) enhanced the release of sAPPalpha in rPCNs treated with IL-1beta or transfected with P2Y(2)R cDNA. UTP-induced release of sAPPalpha from rPCNs was completely inhibited by pre-treatment of the cells with the metalloproteinase inhibitor TACE inhibitor (TAPI-2) or the phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002, and was partially inhibited by the MAPK/extracellular signal-regulated kinase inhibitor U0126 and the protein kinase C inhibitor GF109203. These data suggest that P2Y(2)R-mediated release of sAPPalpha from cortical neurons is directly dependent on a disintegrin and metalloproteinase (ADAM) 10/17 and PI3K activity, whereas extracellular signal-regulated kinase 1/2 and PI3K activity may indirectly regulate APP processing. These results demonstrate that elevated levels of pro-inflammatory cytokines associated with neurodegenerative diseases, such as IL-1beta, can enhance non-amyloidogenic APP processing through up-regulation of the P2Y(2)R in neurons.
Figures













References
-
- Ahn JS, Camden JM, Schrader AM, Redman RS, Turner JT. Reversible regulation of P2Y2 nucleotide receptor expression in the duct-ligated rat submandibular gland. Am J Physiol Cell Physiol. 2000;279:C286–C294. - PubMed
-
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. Review. - PubMed
-
- Allinson TM, Parkin ET, Turner AJ, Hooper NM. ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res. 2003;74:342–352. - PubMed
-
- Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumor-necrosis factor-alpha from cells. Nature. 1997;385:729–733. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

