Microbial factories for recombinant pharmaceuticals
- PMID: 19317892
- PMCID: PMC2669800
- DOI: 10.1186/1475-2859-8-17
Microbial factories for recombinant pharmaceuticals
Abstract
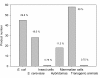
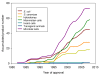
Most of the hosts used to produce the 151 recombinant pharmaceuticals so far approved for human use by the Food and Drug Administration (FDA) and/or by the European Medicines Agency (EMEA) are microbial cells, either bacteria or yeast. This fact indicates that despite the diverse bottlenecks and obstacles that microbial systems pose to the efficient production of functional mammalian proteins, namely lack or unconventional post-translational modifications, proteolytic instability, poor solubility and activation of cell stress responses, among others, they represent convenient and powerful tools for recombinant protein production. The entering into the market of a progressively increasing number of protein drugs produced in non-microbial systems has not impaired the development of products obtained in microbial cells, proving the robustness of the microbial set of cellular systems (so far Escherichia coli and Saccharomyces cerevisae) developed for protein drug production. We summarize here the nature, properties and applications of all those pharmaceuticals and the relevant features of the current and potential producing hosts, in a comparative way.
Figures

References
-
- Manning MC, Patel K, Borchardt RT. Stability of protein pharmaceuticals. Pharm Res. 1989;6:903–918. - PubMed
-
- Human insulin receives FDA approval. FDA Drug Bull. 1982;12:18–19. - PubMed
-
- Redwan e. Cumulative updating of approved biopharmaceuticals. Hum Antibodies. 2007;16:137–158. - PubMed
-
- Gerngross TU. Advances in the production of human therapeutic proteins in yeasts and filamentous fungi. Nat Biotechnol. 2004;22:1409–1414. - PubMed
-
- Schmidt FR. Recombinant expression systems in the pharmaceutical industry. Appl Microbiol Biotechnol. 2004;65:363–372. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

