Designing new cellular signaling pathways
- PMID: 19318206
- PMCID: PMC2661875
- DOI: 10.1016/j.chembiol.2009.01.011
Designing new cellular signaling pathways
Abstract
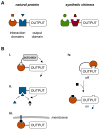
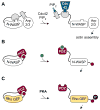
All cells respond to signals from the environment. Extracellular stimuli activate intracellular signal transduction pathways that make decisions about cell identity, behavior, and survival. A nascent field aims to design and construct new signaling pathways beyond those found in nature. Current strategies exploit the structural modularity of many signaling proteins, which makes them inherently amenable to domain-swapping tactics that exchange their input and output connections. The results reveal a remarkable degree of functional plasticity in signaling proteins and pathways, as well as regulatory logic that can be transported to new proteins. Modified adaptor and scaffold proteins can reroute signal traffic and adjust the response behavior of the pathway circuit. These synthetic biology approaches promise to deepen our understanding of existing signaling pathways and spur the development of new cellular tools for research, industry, and medicine.
Figures

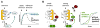
References
-
- Acharya A, Ruvinov SB, Gal J, Moll JR, Vinson C. A heterodimerizing leucine zipper coiled coil system for examining the specificity of a position interactions: amino acids I, V, L, N, A, and K. Biochemistry. 2002;41:14122–14131. - PubMed
-
- Bashor CJ, Helman NC, Yan S, Lim WA. Using engineered scaffold interactions to reshape MAP kinase pathway signaling dynamics. Science. 2008;319:1539–1543. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

