Mitogen-activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana
- PMID: 19318610
- PMCID: PMC2671697
- DOI: 10.1105/tpc.108.062158
Mitogen-activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana
Abstract
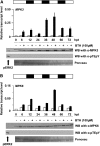
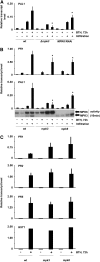
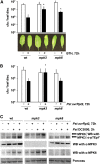
In plants and animals, induced resistance (IR) to biotic and abiotic stress is associated with priming of cells for faster and stronger activation of defense responses. It has been hypothesized that cell priming involves accumulation of latent signaling components that are not used until challenge exposure to stress. However, the identity of such signaling components has remained elusive. Here, we show that during development of chemically induced resistance in Arabidopsis thaliana, priming is associated with accumulation of mRNA and inactive proteins of mitogen-activated protein kinases (MPKs), MPK3 and MPK6. Upon challenge exposure to biotic or abiotic stress, these two enzymes were more strongly activated in primed plants than in nonprimed plants. This elevated activation was linked to enhanced defense gene expression and development of IR. Strong elicitation of stress-induced MPK3 and MPK6 activity is also seen in the constitutive priming mutant edr1, while activity was attenuated in the priming-deficient npr1 mutant. Moreover, priming of defense gene expression and IR were lost or reduced in mpk3 or mpk6 mutants. Our findings argue that prestress deposition of the signaling components MPK3 and MPK6 is a critical step in priming plants for full induction of defense responses during IR.
Figures
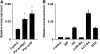


References
-
- Alexander, D., Goodman, R.M., Gut-Rella, M., Glascock, C., Weymann, K., Friedrich, L., Maddox, D., Ahl-Goy, P., Luntz, T., Ward, E., and Ryals, J. (1993). Increased tolerance to two oomycete pathogens in transgenic tobacco expressing pathogenesis-related protein 1a. Proc. Natl. Acad. Sci. USA 90 7327–7331. - PMC - PubMed
-
- Beckers, G.J.M., and Conrath, U. (2007). Priming for stress resistance: from the lab to the field. Curr. Opin. Plant Biol. 10 425–431. - PubMed
-
- Colcombet, J., and Hirt, H. (2008). Arabidopsis MAPKs: A complex signaling network involved in multiple biological processes. Biochem. J. 413 217–226. - PubMed
-
- Conrath, U., et al. (2006). Priming: getting ready for battle. Mol. Plant Microbe Interact. 19 1062–1071. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

