Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer
- PMID: 19318631
- PMCID: PMC2664091
- DOI: 10.1093/jnci/djp030
Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer
Abstract
Background: Overexpression of the fatty acid synthase (FASN) gene has been implicated in prostate carcinogenesis. We sought to directly assess the oncogenic potential of FASN.
Methods: We used immortalized human prostate epithelial cells (iPrECs), androgen receptor-overexpressing iPrECs (AR-iPrEC), and human prostate adenocarcinoma LNCaP cells that stably overexpressed FASN for cell proliferation assays, soft agar assays, and tests of tumor formation in immunodeficient mice. Transgenic mice expressing FASN in the prostate were generated to assess the effects of FASN on prostate histology. Apoptosis was evaluated by Hoechst 33342 staining and by fluorescence-activated cell sorting in iPrEC-FASN cells treated with stimulators of the intrinsic and extrinsic pathways of apoptosis (ie, camptothecin and anti-Fas antibody, respectively) or with a small interfering RNA (siRNA) targeting FASN. FASN expression was compared with the apoptotic index assessed by the terminal deoxynucleotidyltransferase-mediated UTP end-labeling method in 745 human prostate cancer samples by using the least squares means procedure. All statistical tests were two-sided.
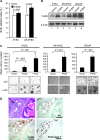
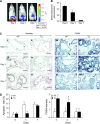
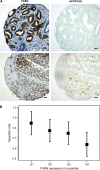
Results: Forced expression of FASN in iPrECs, AR-iPrECs, and LNCaP cells increased cell proliferation and soft agar growth. iPrECs that expressed both FASN and androgen receptor (AR) formed invasive adenocarcinomas in immunodeficient mice (12 of 14 mice injected formed tumors vs 0 of 14 mice injected with AR-iPrEC expressing empty vector (P < .001, Fisher exact test); however, iPrECs that expressed only FASN did not. Transgenic expression of FASN in mice resulted in prostate intraepithelial neoplasia, the incidence of which increased from 10% in 8- to 16-week-old mice to 44% in mice aged 7 months or more (P = .0028, Fisher exact test), but not in invasive tumors. In LNCaP cells, siRNA-mediated silencing of FASN resulted in apoptosis. FASN overexpression protected iPrECs from apoptosis induced by camptothecin but did not protect iPrECs from Fas receptor-induced apoptosis. In human prostate cancer specimens, FASN expression was inversely associated with the apoptotic rate (mean percentage of apoptotic cells, lowest vs highest quartile of FASN expression: 2.76 vs 1.34, difference = 1.41, 95% confidence interval = 0.45 to 2.39, Ptrend = .0046).
Conclusions: These observations suggest that FASN can act as a prostate cancer oncogene in the presence of AR and that FASN exerts its oncogenic effect by inhibiting the intrinsic pathway of apoptosis.
Figures


References
-
- Medes G, Thomas A, Weinhouse S. Metabolism of neoplastic tissue. IV. A study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res. 1953;13(1):27–29. - PubMed
-
- Kusakabe T, Maeda M, Hoshi N, et al. Fatty acid synthase is expressed mainly in adult hormone-sensitive cells or cells with high lipid metabolism and in proliferating fetal cells. J Histochem Cytochem. 2000;48(5):613–622. - PubMed
-
- Shurbaji MS, Kalbfleisch JH, Thurmond TS. Immunohistochemical detection of a fatty acid synthase (OA-519) as a predictor of progression of prostate cancer. Hum Pathol. 1996;27(9):917–921. - PubMed
-
- Alo’ PL, Visca P, Marci A, Mangoni A, Botti C, Di Tondo U. Expression of fatty acid synthase (FAS) as a predictor of recurrence in stage I breast carcinoma patients. Cancer. 1996;77(3):474–482. - PubMed
-
- Gansler TS, Hardman W, 3rd, Hunt DA, Schaffel S, Hennigar RA. Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum Pathol. 1997;28(6):686–692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

