Estimating blood and brain concentrations and blood-to-brain influx by magnetic resonance imaging with step-down infusion of Gd-DTPA in focal transient cerebral ischemia and confirmation by quantitative autoradiography with Gd-[(14)C]DTPA
- PMID: 19319145
- PMCID: PMC4205544
- DOI: 10.1038/jcbfm.2009.20
Estimating blood and brain concentrations and blood-to-brain influx by magnetic resonance imaging with step-down infusion of Gd-DTPA in focal transient cerebral ischemia and confirmation by quantitative autoradiography with Gd-[(14)C]DTPA
Abstract
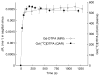
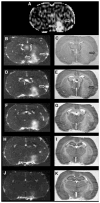
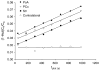
An intravenous step-down infusion procedure that maintained a constant gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA) blood concentration and magnetic resonance imaging (MRI) were used to localize and quantify the blood-brain barrier (BBB) opening in a rat model of transient cerebral ischemia (n=7). Blood-to-brain influx rate constant (K(i)) values of Gd-DTPA from such regions were estimated using MRI-Patlak plots and compared with the K(i) values of Gd-[(14)C]DTPA, determined minutes later in the same rats with an identical step-down infusion, quantitative autoradiography (QAR), and single-time equation. The normalized plasma concentration-time integrals were identical for Gd-DTPA and Gd-[(14)C]DTPA, indicating that the MRI protocol yielded reliable estimates of plasma Gd-DTPA levels. In six rats with a BBB opening, 14 spatially similar regions of extravascular Gd-DTPA enhancement and Gd-[(14)C]DTPA leakage, including one very small area, were observed. The terminal tissue-plasma ratios of Gd-[(14)C]DTPA tended to be slightly higher than those of Gd-DTPA in these regions, but the differences were not significant. The MRI-derived K(i) values for Gd-DTPA closely agreed and correlated well with those obtained for Gd-[(14)C]DTPA. In summary, MRI estimates of Gd-DTPA concentration in the plasma and brain and the influx rate are quantitatively and spatially accurate with step-down infusions.
Conflict of interest statement
The authors state no conflict of interest.
Figures

Similar articles
-
MRI and quantitative autoradiographic studies following bolus injections of unlabeled and (14)C-labeled gadolinium-diethylenetriaminepentaacetic acid in a rat model of stroke yield similar distribution volumes and blood-to-brain influx rate constants.NMR Biomed. 2011 Jun;24(5):547-58. doi: 10.1002/nbm.1625. Epub 2010 Dec 12. NMR Biomed. 2011. PMID: 21674656 Free PMC article.
-
Quantitation and localization of blood-to-brain influx by magnetic resonance imaging and quantitative autoradiography in a model of transient focal ischemia.Magn Reson Med. 2005 Oct;54(4):813-21. doi: 10.1002/mrm.20629. Magn Reson Med. 2005. PMID: 16142715
-
Step-down infusions of Gd-DTPA yield greater contrast-enhanced magnetic resonance images of BBB damage in acute stroke than bolus injections.Magn Reson Imaging. 2007 Apr;25(3):311-8. doi: 10.1016/j.mri.2006.09.003. Epub 2006 Nov 7. Magn Reson Imaging. 2007. PMID: 17371719 Free PMC article.
-
Patlak plots of Gd-DTPA MRI data yield blood-brain transfer constants concordant with those of 14C-sucrose in areas of blood-brain opening.Magn Reson Med. 2003 Aug;50(2):283-92. doi: 10.1002/mrm.10524. Magn Reson Med. 2003. PMID: 12876704
-
Acute blood-brain barrier opening in experimentally induced focal cerebral ischemia is preferentially identified by quantitative magnetization transfer imaging.Magn Reson Med. 2005 Oct;54(4):822-32. doi: 10.1002/mrm.20630. Magn Reson Med. 2005. PMID: 16142716
Cited by
-
High and Low Molecular Weight Fluorescein Isothiocyanate (FITC)-Dextrans to Assess Blood-Brain Barrier Disruption: Technical Considerations.Transl Stroke Res. 2011 Mar;2(1):106-11. doi: 10.1007/s12975-010-0049-x. Epub 2010 Nov 11. Transl Stroke Res. 2011. PMID: 21423333 Free PMC article.
-
Extravasation into brain and subsequent spread beyond the ischemic core of a magnetic resonance contrast agent following a step-down infusion protocol in acute cerebral ischemia.Fluids Barriers CNS. 2014 Sep 23;11:21. doi: 10.1186/2045-8118-11-21. eCollection 2014. Fluids Barriers CNS. 2014. PMID: 25276343 Free PMC article.
-
In vivo methods to study uptake of nanoparticles into the brain.Pharm Res. 2011 Mar;28(3):456-71. doi: 10.1007/s11095-010-0291-7. Epub 2010 Oct 7. Pharm Res. 2011. PMID: 20924653 Free PMC article. Review.
-
MRI and quantitative autoradiographic studies following bolus injections of unlabeled and (14)C-labeled gadolinium-diethylenetriaminepentaacetic acid in a rat model of stroke yield similar distribution volumes and blood-to-brain influx rate constants.NMR Biomed. 2011 Jun;24(5):547-58. doi: 10.1002/nbm.1625. Epub 2010 Dec 12. NMR Biomed. 2011. PMID: 21674656 Free PMC article.
-
Model selection in measures of vascular parameters using dynamic contrast-enhanced MRI: experimental and clinical applications.NMR Biomed. 2013 Aug;26(8):1028-41. doi: 10.1002/nbm.2996. NMR Biomed. 2013. PMID: 23881857 Free PMC article. Review.
References
-
- Ba-Ssalamah A, Nobauer-Huhmann IM, Pinker K, Schibany N, Prokesch R, Mehrain S, Mlynarik V, Fog A, Heimberger K, Trattnig S. Effect of contrast dose and field strength in the magnetic resonance detection of brain metastases. Invest Radiol. 2003;38:415–22. - PubMed
-
- Blasberg R, Patlak CS, Fenstermacher JD. Selection of experimental conditions for the accurate determination of blood-brain transfer constants from single-time experiments: a theoretical analysis. J Cereb Blood Flow Metab. 1983;3:215–25. - PubMed
-
- Bruening R, Berchtenbreiter C, Holzknecht N, Essig M, Wu RH, Simmons A, Heuck A, Maschek A, Meusel M, Williams SC, Cox T, Knopp MV, Reiser M. Effects of three different doses of a bolus injection of gadodiamide: assessment of regional cerebral blood volume maps in a blinded reader study.[see comment] Ajnr Am J Neuroradiol. 2000;21:1603–10. - PMC - PubMed
-
- Carson RE, Channing MA, Blasberg RG, Dunn BB, Cohen RM, Rice KC, Herscovitch P. Comparison of bolus and infusion methods for receptor quantitation: application to [18F]cyclofoxy and positron emission tomography. J Cereb Blood Flow Metab. 1993;13:24–42. - PubMed
-
- Correale J, Villa A. The blood-brain barrier in multiple sclerosis: functional roles and therapeutic targeting. Autoimmunity. 2007;40:148–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

