Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha
- PMID: 19319200
- PMCID: PMC2657211
- DOI: 10.1371/journal.pone.0004942
Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha
Abstract
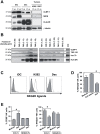
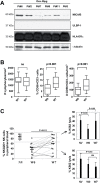
Dendritic cell (DC) derived-exosomes (Dex) are nanomeric vesicles harboring functional MHC/peptide complexes promoting T cell-dependent tumor rejection. In the first Phase I trial using peptide-pulsed Dex, the observation of clinical regressions in the absence of T cell responses prompted the search for alternate effector mechanisms. Mouse studies unraveled the bioactivity of Dex on NK cells. Indeed, Dex promoted an IL-15Ralpha- and NKG2D-dependent NK cell proliferation and activation respectively, resulting in anti-metastatic effects mediated by NK1.1(+) cells. In humans, Dex express functional IL-15Ralpha which allow proliferation and IFNgamma secretion by NK cells. In contrast to immature DC, human Dex harbor NKG2D ligands on their surface leading to a direct engagement of NKG2D and NK cell activation ex vivo. In our phase I clinical trial, we highlight the capacity of Dex based-vaccines to restore the number and NKG2D-dependent function of NK cells in 7/14 patients. Altogether, these data provide a mechanistic explanation on how Dex may stimulate non MHC restricted-anti-tumor effectors and induce tumor regression in vivo.
Conflict of interest statement
Figures


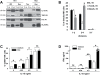
References
-
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. - PubMed
-
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. - PubMed
-
- Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. - PubMed
-
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. - PubMed
-
- Pardoll DM. Cancer vaccines. Nat Med. 1998;4:525–531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

