The H2 receptor antagonist nizatidine is a P-glycoprotein substrate: characterization of its intestinal epithelial cell efflux transport
- PMID: 19319690
- PMCID: PMC2691456
- DOI: 10.1208/s12248-009-9092-5
The H2 receptor antagonist nizatidine is a P-glycoprotein substrate: characterization of its intestinal epithelial cell efflux transport
Abstract
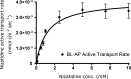
The aim of this study was to elucidate the intestinal epithelial cell efflux transport processes that are involved in the intestinal transport of the H(2) receptor antagonist nizatidine. The intestinal epithelial efflux transport mechanisms of nizatidine were investigated and characterized across Caco-2 cell monolayers, in the concentration range 0.05-10 mM in both apical-basolateral (AP-BL) and BL-AP directions, and the transport constants of P-glycoprotein (P-gp) efflux activity were calculated. The concentration-dependent effects of various P-gp (verapamil, quinidine, erythromycin, ketoconazole, and cyclosporine A), multidrug resistant-associated protein 2 (MRP2; MK-571, probenecid, indomethacin, and p-aminohipuric acid), and breast cancer resistance protein (BCRP; Fumitremorgin C) inhibitors on nizatidine bidirectional transport were examined. Nizatidine exhibited 7.7-fold higher BL-AP than AP-BL Caco-2 permeability, indicative of net mucosal secretion. All P-gp inhibitors investigated displayed concentration-dependent inhibition on nizatidine secretion in both directions. The IC(50) of verapamil on nizatidine P-gp secretion was 1.2 x 10(-2) mM. In the absence of inhibitors, nizatidine displayed concentration-dependent secretion, with one saturable (J(max) = 5.7 x 10(-3) nmol cm(-2) s(-1) and K(m) = 2.2 mM) and one nonsaturable component (K(d) = 7 x 10(-4) microL cm(-2) s(-1)). Under complete P-gp inhibition, nizatidine exhibited linear secretory flux, with a slope similar to the nonsaturable component. V(max) and K(m) estimated for nizatidine P-gp-mediated secretion were 4 x 10(-3) nmol cm(-2) s(-1) and 1.2 mM, respectively. No effect was obtained with the MRP2 or the BCRP inhibitors. Being a drug commonly used in pediatrics, adults, and elderly, nizatidine susceptibility to efflux transport by P-gp revealed in this paper may be of significance in its absorption, distribution, and clearance, as well as possible drug-drug interactions.
Figures





Similar articles
-
Small intestinal efflux mediated by MRP2 and BCRP shifts sulfasalazine intestinal permeability from high to low, enabling its colonic targeting.Am J Physiol Gastrointest Liver Physiol. 2009 Aug;297(2):G371-7. doi: 10.1152/ajpgi.00102.2009. Epub 2009 Jun 18. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19541926
-
Multiple efflux pumps are involved in the transepithelial transport of colchicine: combined effect of p-glycoprotein and multidrug resistance-associated protein 2 leads to decreased intestinal absorption throughout the entire small intestine.Drug Metab Dispos. 2009 Oct;37(10):2028-36. doi: 10.1124/dmd.109.028282. Epub 2009 Jul 9. Drug Metab Dispos. 2009. PMID: 19589874
-
Role of P-glycoprotein in the intestinal absorption of tanshinone IIA, a major active ingredient in the root of Salvia miltiorrhiza Bunge.Curr Drug Metab. 2007 May;8(4):325-40. doi: 10.2174/138920007780655450. Curr Drug Metab. 2007. PMID: 17504222
-
Genetic Predictors of Antipsychotic Efflux Impairment via Blood-Brain Barrier: Role of Transport Proteins.Genes (Basel). 2023 May 15;14(5):1085. doi: 10.3390/genes14051085. Genes (Basel). 2023. PMID: 37239445 Free PMC article. Review.
-
Investigating Intestinal Transporter Involvement in Rivaroxaban Disposition through Examination of Changes in Absorption.Pharm Res. 2021 May;38(5):795-801. doi: 10.1007/s11095-021-03039-3. Epub 2021 Apr 13. Pharm Res. 2021. PMID: 33847849 Free PMC article.
Cited by
-
Provisional in-silico biopharmaceutics classification (BCS) to guide oral drug product development.Drug Des Devel Ther. 2014 Sep 24;8:1563-75. doi: 10.2147/DDDT.S68909. eCollection 2014. Drug Des Devel Ther. 2014. PMID: 25284986 Free PMC article.
-
Advantageous Solubility-Permeability Interplay When Using Amorphous Solid Dispersion (ASD) Formulation for the BCS Class IV P-gp Substrate Rifaximin: Simultaneous Increase of Both the Solubility and the Permeability.AAPS J. 2017 May;19(3):806-813. doi: 10.1208/s12248-017-0052-1. Epub 2017 Feb 15. AAPS J. 2017. PMID: 28204967
-
Oral delivery of lipophilic drugs: the tradeoff between solubility increase and permeability decrease when using cyclodextrin-based formulations.PLoS One. 2013 Jul 16;8(7):e68237. doi: 10.1371/journal.pone.0068237. Print 2013. PLoS One. 2013. PMID: 23874557 Free PMC article.
-
Prediction of solubility and permeability class membership: provisional BCS classification of the world's top oral drugs.AAPS J. 2009 Dec;11(4):740-6. doi: 10.1208/s12248-009-9144-x. Epub 2009 Oct 30. AAPS J. 2009. PMID: 19876745 Free PMC article. Review.
-
Renal Drug Transporters and Drug Interactions.Clin Pharmacokinet. 2017 Aug;56(8):825-892. doi: 10.1007/s40262-017-0506-8. Clin Pharmacokinet. 2017. PMID: 28210973 Review.
References
-
- Orenstein S. R., Gremse D. A., Pantaleon C. D., Kling D. F., Rotenberg K. S. Nizatidine for the treatment of pediatric gastroesophageal reflux symptoms: An open-label, multiple-dose, randomized, multicenter clinical trial in 210 children. Clin. Ther. 2005;27:472. doi: 10.1016/j.clinthera.2005.04.008. - DOI - PubMed
-
- Sasaki M., Sudoh T., Fujimura A. Pharmacokinetics of ranitidine and nizatidine in very elderly patients. Am. J. Ther. 2005;12:223–225. - PubMed
-
- Feldman M., Burton M. Histamine2-receptor antagonists. Standard therapy for acid-peptic diseases. 1. N. Engl. J. Med. 1990;323:1672–1680. - PubMed
-
- Knadler M. P., Bergstrom R. F., Callaghan J. T., Rubin A. Nizatidine, an H2-blocker. Its metabolism and disposition in man. Drug Metab. Dispos. 1986;14:175–182. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

