Biotin
- PMID: 19319844
- PMCID: PMC4757853
- DOI: 10.1002/biof.8
Biotin
Abstract
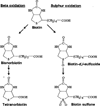
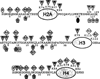
Biotin is a water-soluble vitamin and serves as a coenzyme for five carboxylases in humans. Biotin is also covalently attached to distinct lysine residues in histones, affecting chromatin structure and mediating gene regulation. This review describes mammalian biotin metabolism, biotin analysis, markers of biotin status, and biological functions of biotin. Proteins such as holocarboxylase synthetase, biotinidase, and the biotin transporters SMVT and MCT1 play crucial roles in biotin homeostasis, and these roles are reviewed here. Possible effects of inadequate biotin intake, drug interactions, and inborn errors of metabolism are discussed, including putative effects on birth defects.
(c) 2009 International Union of Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Kogl F, Tonnis B. Über das Bios-Problem. Darstellung von krystallisiertem Biotin aus Eigelb. Z. Physiol Chem. 1932;242:43–73.
-
- du Vigneaud V, Melville DB, Folkers K, Wolf DE, Mozingo DE, Keresztesy JC, Harris SA. The structure of biotin: a study of desthiobiotin. J. Biol. Chem. 1942;146:475–485.
-
- Harris SA, Wolf DE, Mozingo R, Folkers K. Synthetic biotin. Science. 1943;97:447–448. - PubMed
-
- Hatakeyama K, Kobayashi M, Yukawa H. Analysis of biotin biosynthesis pathway in coryneform bacteria: Brevibacterium flavum. In: McCormick DB, Suttie JW, Wagner C, editors. Vitamins and Coenzymes, Part I. San Diego, CA: Academic Press; 1997. pp. 339–348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

