Real-time quantitative PCR for analysis of candidate fungal biopesticides against malaria: technique validation and first applications
- PMID: 19320043
- PMCID: PMC2666797
- DOI: 10.1016/j.jip.2009.01.006
Real-time quantitative PCR for analysis of candidate fungal biopesticides against malaria: technique validation and first applications
Abstract
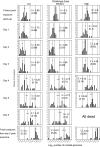
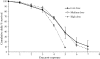
Recent research has indicated that fungal biopesticides could augment existing malaria vector control tools. Here we present a set of methodologies to monitor the in vivo kinetics of entomopathogenic fungi in Anopheles in the presence or absence of malaria parasites using quantitative real-time PCR. Three qPCR assays were successfully developed for counting fungal genomes: "specific" assays capable of distinguishing two well characterized fungal entomopathogens Beauveria bassiana isolate IMI391510 and Metarhizium anisopliae var. acridum isolate IMI330189, both of which have previously been shown to be virulent to Anopheles mosquitoes, and a "generic" fungal assay for determining any fungal burden. A fourth assay to Plasmodium chabaudi enabled quantification of co-infecting malarial parasites. All qPCR assays provide sensitive, target-specific, and robust quantification over a linear range of greater than five orders of magnitude (seven orders of magnitude for the fungal assays). B. bassiana growth within mosquitoes exposed to three different conidial challenge doses was monitored using the B. bassiana-specific assay and represents the first description of entomopathogenic fungal replication within an insect host. This revealed that, irrespective of challenge dose, after several days of relatively little replication, a sudden on-set of substantial nuclear division occurs, accompanied by physical fungal growth (hyphae) within the mosquito haemocoel shortly before death. Exposure to higher densities of conidia resulted in significantly greater pick-up by mosquitoes and to elevated fungal burdens at each time point sampled. High fungal burdens, comparable to those identified in cadavers, were attained more rapidly and mortalities occurred earlier post-exposure with increasing challenge dose. The lines of research made possible by the qPCR assays described here will contribute to optimization of fungal biopesticides against malaria and other vector-borne diseases.
Figures



References
-
- Bell A.S., Ranford-Cartwright L. A real-time PCR assay for quantifying Plasmodium falciparum infections in the mosquito vector. Int. J. Parasitol. 2004;34:795–802. - PubMed
-
- Bell A.S., de Roode J.C., Sim D., Read A.F. Within-host competition in genetically diverse malaria infections: parasite virulence and competitive success. Evolution. 2006;60(7):1358–1371. - PubMed
-
- Blanford S., Chan B.H.K., Jenkins N., Sim D., Turner R.J., Read A.F., Thomas M.B. Fungal pathogen reduces potential for malaria transmission. Science. 2005;308:1638–1641. - PubMed
-
- Castrillo L.A., Vandenberg J.D., Wraight S.P. Strain-specific detection of introduced Beauveria bassiana in agricultural fields by use of sequence-characterized amplified region markers. J. Invertebr. Pathol. 2003;82(2):75–83. - PubMed
-
- Castrillo L.A., Griggs M.H., Vandenberg J.D. Quantitative detection of Beauveria bassiana GHA (Ascomycota: Hypocreales), a potential microbial control agent of the emerald ash borer, by use of real-time PCR. Biol. Control. 2008;45:163–169.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

