Extracellular dopamine and norepinephrine in the developing rat prefrontal cortex: transient effects of early partial loss of dopamine
- PMID: 19320060
- PMCID: PMC2745404
- DOI: 10.1016/j.brainresbull.2009.01.012
Extracellular dopamine and norepinephrine in the developing rat prefrontal cortex: transient effects of early partial loss of dopamine
Abstract
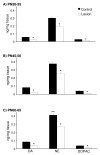
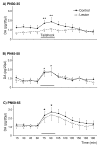
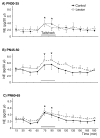
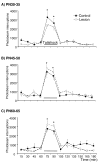
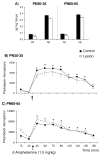
Early developmental abnormalities affecting mesocortical dopamine (DA) neurons may result in later functional deficits that play a role in the emergence of psychiatric illness in adolescence/early adulthood. Little is known about the functional maturation of these neurons under either normal or abnormal conditions. In the present study, 6-hydroxydopamine was infused into the rat medial prefrontal cortex (mPFC) on postnatal day (PN) 12-14. On PN30-35, 45-50, and 60-65, mPFC extracellular DA and norepinephrine (NE) concentrations were monitored in intact and lesioned rats using in vivo microdialysis. Extracellular DA and NE concentrations in the intact mPFC remain fairly stable across development; one exception being a trend for acute tailshock-evoked DA concentrations to increase as a function of age. Lesioned rats sustained a persistent (approximately 50%) decrease in mPFC tissue DA concentrations. Tailshock-evoked increases in mPFC extracellular DA were attenuated in lesioned rats tested on PN30-35, but not PN45-50 or 60-65. Basal and evoked extracellular NE was unaffected in lesioned rats tested at any age, despite a persistent (approximately 25%) decrease in tissue NE content. Horizontal locomotor activity was also assessed in the present study. Results of previous studies suggest this behavior is modulated by mesoprefrontal DA neurons. Although not significant, acute tailshock- and acute amphetamine-evoked horizontal locomotor activity tended to be attenuated in lesioned rats tested on PN30-35 and augmented in lesioned rats tested on PN60-65. The present data suggest that early partial loss of mesoprefrontal DA nerve terminals, resulting in a persistent decrease in tissue DA concentrations, is unlikely to result in persistent alterations in local DA release.
Conflict of interest statement
Figures
Similar articles
-
Neonatal depletion of cortical dopamine: effects on dopamine turnover and motor behavior in juvenile and adult rats.Brain Res Dev Brain Res. 2005 May 12;156(2):167-75. doi: 10.1016/j.devbrainres.2005.02.006. Brain Res Dev Brain Res. 2005. PMID: 16099303
-
Effects of selective dopamine depletion in medial prefrontal cortex on basal and evoked extracellular dopamine in neostriatum.Brain Res. 1995 Jul 10;685(1-2):117-28. doi: 10.1016/0006-8993(95)00421-l. Brain Res. 1995. PMID: 7583236
-
Destruction of noradrenergic terminals increases dopamine concentration and reduces dopamine metabolism in the medial prefrontal cortex.Behav Brain Res. 2018 May 15;344:57-64. doi: 10.1016/j.bbr.2018.02.016. Epub 2018 Feb 14. Behav Brain Res. 2018. PMID: 29454007
-
Noradrenergic terminals are the primary source of α2-adrenoceptor mediated dopamine release in the medial prefrontal cortex.Prog Neuropsychopharmacol Biol Psychiatry. 2019 Mar 2;90:97-103. doi: 10.1016/j.pnpbp.2018.11.015. Epub 2018 Nov 23. Prog Neuropsychopharmacol Biol Psychiatry. 2019. PMID: 30472147
-
Local influence of endogenous norepinephrine on extracellular dopamine in rat medial prefrontal cortex.J Neurochem. 1995 Jul;65(1):111-6. doi: 10.1046/j.1471-4159.1995.65010111.x. J Neurochem. 1995. PMID: 7790854
Cited by
-
Atomoxetine facilitates attentional set shifting in adolescent rats.Dev Cogn Neurosci. 2011 Oct;1(4):552-9. doi: 10.1016/j.dcn.2011.04.003. Dev Cogn Neurosci. 2011. PMID: 21927630 Free PMC article.
-
Animal Models of Relevance to the Schizophrenia Prodrome.Biol Psychiatry Glob Open Sci. 2021 Dec 9;3(1):22-32. doi: 10.1016/j.bpsgos.2021.12.001. eCollection 2023 Jan. Biol Psychiatry Glob Open Sci. 2021. PMID: 36712558 Free PMC article. Review.
-
Effects of age and acute ethanol on glutamatergic neurotransmission in the medial prefrontal cortex of freely moving rats using enzyme-based microelectrode amperometry.PLoS One. 2015 Apr 30;10(4):e0125567. doi: 10.1371/journal.pone.0125567. eCollection 2015. PLoS One. 2015. PMID: 25927237 Free PMC article.
-
Severe Myoclonic Epilepsy in Infancy - Adult Phenotype with Bradykinesia, Hypomimia, and Perseverative Behavior: Report of Five Cases.Mol Syndromol. 2010;1(5):231-238. doi: 10.1159/000326746. Epub 2011 Mar 26. Mol Syndromol. 2010. PMID: 22140375 Free PMC article.
-
Enhanced brain-derived neurotrophic factor signaling in the nucleus accumbens of juvenile rats.Dev Neurosci. 2013;35(5):384-95. doi: 10.1159/000351026. Epub 2013 Sep 7. Dev Neurosci. 2013. PMID: 24021607 Free PMC article.
References
-
- Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry. 1999;156:1580–9. - PubMed
-
- Andersen SL, Dumont NL, Teicher MH. Developmental differences in dopamine synthesis inhibition by (+/−)-7-OH-DPAT. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:173–81. - PubMed
-
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–9. - PubMed
-
- Arnsten AF. Stimulants: Therapeutic Actions in ADHD. Neuropsychopharmacology. 2006 - PubMed
-
- Banks K, Gratton A. Possible involvement of medial prefrontal cortex in amphetamine-induced sensitization of mesolimbic dopamine function. European Journal of Pharmacology. 1995;282:157–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

