Single-flask synthesis of N-acylated indoles by catalytic dehydrogenative coupling with primary alcohols
- PMID: 19320508
- PMCID: PMC2782717
- DOI: 10.1021/ol900306v
Single-flask synthesis of N-acylated indoles by catalytic dehydrogenative coupling with primary alcohols
Abstract
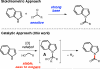
Indoles and alcohols can be coupled in a dehydrogenative process catalyzed by tetrapropylammonium perruthenate. This efficient approach to indolylamides proceeds in a single flask under mild conditions. By employing substituted indoles and alkyl, branched, or benzylic alcohols, a variety of indolylamides can be formed. Aryl indolylamides can be functionalized through an additional dehydrogenative coupling to furnish elaborated polycyclic heterocycles similar to biologically active structures previously reported.
Figures
References
-
- Humphrey JM, Chamberlin AR. Chem. Rev. 1997;97:2243–2266. - PubMed
-
- Larock RC. Comprehensive Organic Transformations. 2nd ed. Wiley-VCH; New York: 1999.
-
- Yoo WJ, Li CJ. J. Am. Chem. Soc. 2006;128:13064–13065. - PubMed
- Suto Y, Yamagiwa N, Torisawa Y. Tetrahedron Lett. 2008;49:5732–5735.
- Fang C, Qian WX, Bao WL. Synlett. 2008:2529–2531.
- Colombeau L, Traore T, Compain P, Martin OR. J. Org. Chem. 2008;73:8647–8650. - PubMed
- Chan J, Baucorn KD, Murry JA. J. Am. Chem. Soc. 2007;129:14106–14107. - PubMed
-
(f) The oxidative amidation of aldehydes has been recently reviewed: Ekoue-Kovi K, Wolf C. Chem.-Eur. J. 2008;14:6302–6315..
-
-
For examples of carboxylic acids protected as indolylamides, see (a) ref. 9a De Oliveira Baptista MJV, Barrett AGM, Barton DHR, Girijavallabhan M, Jennings RC, Kelly J, Papadimitriou VJ, Turner JV, Usher NA. J. Chem. Soc., Perkin Trans. 1977;1:1477–1500.. Barrett AGM, Dhanak D. Tetrahedron Lett. 1987;28:3327–3330.. Barrett AGM, Bezuidenhoudt BCB, Dhanak D, Gasiecki AF, Howell AR, Lee AC, Russell MA. J. Org. Chem. 1989;54:3321–3324.. Buller MJ, Gilley CB, Nguyen B, Olshansky L, Fraga B, Kobayashi Y. Synlett. 2008:2244–2248.. Isaacson J, Loo M, Kobayashi Y. Org. Lett. 2008;10:1461–1463.. Linda P, Stener A, Cipiciani A, Savelli G. J. Heterocycl. Chem. 1983;20:247–248..
-
-
- Shen TY, Lucas S, Sarett LH, Rosegray A, Nuss GW, Willett JD, Ellis RL, Holly FW, Matzuk AR, Wilson AN, Winter CA, Windholz TB, Risley EA, Stammer CH, Holtz WJ, Witzel BE. J. Am. Chem. Soc. 1963;85:488–489.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources