Mutagenesis studies toward understanding the intracellular signaling mechanism of antithrombin
- PMID: 19320820
- PMCID: PMC2720322
- DOI: 10.1111/j.1538-7836.2009.03337.x
Mutagenesis studies toward understanding the intracellular signaling mechanism of antithrombin
Abstract
Summary background: Recent studies have indicated that antithrombin (AT) possesses both anti-inflammatory and antiangiogenic properties.
Objectives: The purpose of this study was to investigate the mechanism of the intracellular signaling activities of AT using wild-type and mutant serpins that have reduced anticoagulant activities due to mutations in either the reactive center loop (RCL) or the heparin-binding site.
Methods: Direct cellular effects of the AT derivatives were compared in the LPS-stimulated endothelial cells by employing permeability and neutrophil adhesion assays in the absence and presence of pertussis toxin (PTX) and siRNAs for either syndecan-4 or sphingosine 1-phosphate receptor 1 (S1P(1)). Furthermore, the roles of prostacyclin and nuclear factor (NF)-kappaB in modulating these effects were investigated.
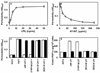
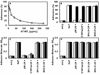
Results: Both wild-type and the RCL mutant, AT/Proth-2, exhibited similar potent barrier protective activities and inhibited the adhesion of neutrophils to endothelial cells via inhibition of the NF-kappaB pathway. Indomethacin abrogated both activities. The heparin-binding site mutants, AT-K114E and AT-K125E, did not exhibit any protective activity in either one of these assays, but a potent pro-apoptotic activity was observed for the AT-K114E in endothelial cells. Both PTX and siRNA for syndecan-4 inhibited the protective effect of AT, but the siRNA for S1P(1) was inconsequential.
Conclusions: The interaction of AT with syndecan-4 is required for its prostacyclin-dependent protective effect through a PTX-sensitive and non-S1P(1)-related G(i)-protein coupled receptor. The RCL mutant, AT/Proth-2, with a markedly reduced anticoagulant but normal protective signaling properties, may potentially be developed as a safer anti-inflammatory drug without increasing the risk of bleeding.
Figures






Similar articles
-
Photolysis of caged sphingosine-1-phosphate induces barrier enhancement and intracellular activation of lung endothelial cell signaling pathways.Am J Physiol Lung Cell Mol Physiol. 2011 Jun;300(6):L840-50. doi: 10.1152/ajplung.00404.2010. Epub 2011 Apr 8. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21478254 Free PMC article.
-
Syndecan-4-dependent signaling in the inhibition of endotoxin-induced endothelial adherence of neutrophils by antithrombin.Thromb Haemost. 2003 Dec;90(6):1150-7. doi: 10.1160/TH03-03-0184. Thromb Haemost. 2003. PMID: 14652650
-
Antithrombin is protective against myocardial ischemia and reperfusion injury.J Thromb Haemost. 2013 Jun;11(6):1020-8. doi: 10.1111/jth.12243. J Thromb Haemost. 2013. PMID: 23582062 Free PMC article.
-
Sphingosine-1-phosphate signaling in physiology and diseases.Biofactors. 2012 Sep-Oct;38(5):329-37. doi: 10.1002/biof.1030. Epub 2012 Jun 7. Biofactors. 2012. PMID: 22674845 Review.
-
Molecular mechanisms of antithrombin-heparin regulation of blood clotting proteinases. A paradigm for understanding proteinase regulation by serpin family protein proteinase inhibitors.Biochimie. 2010 Nov;92(11):1587-96. doi: 10.1016/j.biochi.2010.05.011. Epub 2010 Jun 2. Biochimie. 2010. PMID: 20685328 Free PMC article. Review.
Cited by
-
Antithrombin protects against Plasmodium falciparum histidine-rich protein II-mediated inflammation and coagulation.Blood Adv. 2022 Feb 8;6(3):931-945. doi: 10.1182/bloodadvances.2021005836. Blood Adv. 2022. PMID: 34768285 Free PMC article.
-
Mediation effect of antithrombin III between chronic renal insufficiency and chronic coronary artery disease in T2DM patients.Endocrine. 2024 Jun;84(3):924-933. doi: 10.1007/s12020-023-03669-0. Epub 2024 Jan 8. Endocrine. 2024. PMID: 38190026
-
Thr90Ser Mutation in Antithrombin is Associated with Recurrent Thrombosis in a Heterozygous Carrier.Thromb Haemost. 2020 Jul;120(7):1045-1055. doi: 10.1055/s-0040-1710590. Epub 2020 May 18. Thromb Haemost. 2020. PMID: 32422680 Free PMC article.
-
Expression and functional characterization of two natural heparin-binding site variants of antithrombin.J Thromb Haemost. 2018 Feb;16(2):330-341. doi: 10.1111/jth.13920. Epub 2018 Jan 8. J Thromb Haemost. 2018. PMID: 29215785 Free PMC article.
-
Extracellular Histones Bind Vascular Glycosaminoglycans and Inhibit the Anti-Inflammatory Function of Antithrombin.Cell Physiol Biochem. 2021 Oct 16;55(5):605-617. doi: 10.33594/000000438. Cell Physiol Biochem. 2021. PMID: 34655467 Free PMC article.
References
-
- Gettins PGW. Serpin structure, mechanism, and function. Chem Rev. 2002;102:4751–4803. - PubMed
-
- Marcum JA, Rosenberg RD. Anticoagulantly active heparin-like molecules from the vascular tissue. Biochemistry. 1984;23:1730–1737. - PubMed
-
- Pratt CW, Whinna HC, Church FC. A comparison of three heparin-binding serine proteinase inhibitors. J Biol Chem. 1992;267:8795–8801. - PubMed
-
- Belzar KJ, Zhou A, Carrell RW, Gettins PGW, Huntington JA. Helix D elongation and allosteric activation of antithrombin. J Biol Chem. 2002;277:8551–8558. - PubMed
-
- Schedin-Weiss S, Arocas V, Bock SC, Olson ST, Björk I. Specificity of the basic side chains of Lys114, Lys125, and Arg129 of antithrombin in heparin binding. Biochemistry. 2002;41:12369–12376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

