Mutagenesis studies toward understanding the intracellular signaling mechanism of antithrombin
- PMID: 19320820
- PMCID: PMC2720322
- DOI: 10.1111/j.1538-7836.2009.03337.x
Mutagenesis studies toward understanding the intracellular signaling mechanism of antithrombin
Abstract
Summary background: Recent studies have indicated that antithrombin (AT) possesses both anti-inflammatory and antiangiogenic properties.
Objectives: The purpose of this study was to investigate the mechanism of the intracellular signaling activities of AT using wild-type and mutant serpins that have reduced anticoagulant activities due to mutations in either the reactive center loop (RCL) or the heparin-binding site.
Methods: Direct cellular effects of the AT derivatives were compared in the LPS-stimulated endothelial cells by employing permeability and neutrophil adhesion assays in the absence and presence of pertussis toxin (PTX) and siRNAs for either syndecan-4 or sphingosine 1-phosphate receptor 1 (S1P(1)). Furthermore, the roles of prostacyclin and nuclear factor (NF)-kappaB in modulating these effects were investigated.
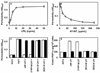
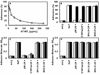
Results: Both wild-type and the RCL mutant, AT/Proth-2, exhibited similar potent barrier protective activities and inhibited the adhesion of neutrophils to endothelial cells via inhibition of the NF-kappaB pathway. Indomethacin abrogated both activities. The heparin-binding site mutants, AT-K114E and AT-K125E, did not exhibit any protective activity in either one of these assays, but a potent pro-apoptotic activity was observed for the AT-K114E in endothelial cells. Both PTX and siRNA for syndecan-4 inhibited the protective effect of AT, but the siRNA for S1P(1) was inconsequential.
Conclusions: The interaction of AT with syndecan-4 is required for its prostacyclin-dependent protective effect through a PTX-sensitive and non-S1P(1)-related G(i)-protein coupled receptor. The RCL mutant, AT/Proth-2, with a markedly reduced anticoagulant but normal protective signaling properties, may potentially be developed as a safer anti-inflammatory drug without increasing the risk of bleeding.
Figures






References
-
- Gettins PGW. Serpin structure, mechanism, and function. Chem Rev. 2002;102:4751–4803. - PubMed
-
- Marcum JA, Rosenberg RD. Anticoagulantly active heparin-like molecules from the vascular tissue. Biochemistry. 1984;23:1730–1737. - PubMed
-
- Pratt CW, Whinna HC, Church FC. A comparison of three heparin-binding serine proteinase inhibitors. J Biol Chem. 1992;267:8795–8801. - PubMed
-
- Belzar KJ, Zhou A, Carrell RW, Gettins PGW, Huntington JA. Helix D elongation and allosteric activation of antithrombin. J Biol Chem. 2002;277:8551–8558. - PubMed
-
- Schedin-Weiss S, Arocas V, Bock SC, Olson ST, Björk I. Specificity of the basic side chains of Lys114, Lys125, and Arg129 of antithrombin in heparin binding. Biochemistry. 2002;41:12369–12376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

