Increased peripheral nerve excitability and local NaV1.8 mRNA up-regulation in painful neuropathy
- PMID: 19320998
- PMCID: PMC2667430
- DOI: 10.1186/1744-8069-5-14
Increased peripheral nerve excitability and local NaV1.8 mRNA up-regulation in painful neuropathy
Abstract
Background: Neuropathic pain caused by peripheral nerve injury is a chronic disorder that represents a significant clinical challenge because the pathological mechanisms have not been fully elucidated. Several studies have suggested the involvement of various sodium channels, including tetrodotoxin-resistant NaV1.8, in affected dorsal root ganglion (DRG) neurons. We have hypothesized that altered local expression of NaV1.8 in the peripheral axons of DRG neurons could facilitate nociceptive signal generation and propagation after neuropathic injury.
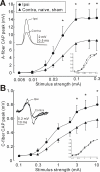
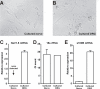
Results: After unilateral sciatic nerve entrapment injury in rats, compound action potential amplitudes were increased in both myelinated and unmyelinated fibers of the ipsilateral sciatic nerve. Tetrodotoxin resistance of both fiber populations and sciatic nerve NaV1.8 immunoreactivity were also increased. Further analysis of NaV1.8 distribution revealed that immunoreactivity and mRNA levels were decreased and unaffected, respectively, in the ipsilateral L4 and L5 DRG; however sciatic nerve NaV1.8 mRNA showed nearly an 11-fold ipsilateral increase. Nav1.8 mRNA observed in the sciatic nerve was likely of axonal origin since it was not detected in non-neuronal cells cultured from nerve tissue. Absence of changes in NaV1.8 mRNA polyadenylation suggests that increased mRNA stability was not responsible for the selective peripheral mRNA increase. Furthermore, mRNA levels of NaV1.3, NaV1.5, NaV1.6, NaV1.7, and NaV1.9 were not significantly different between ipsilateral and contralateral nerves. We therefore propose that selective NaV1.8 mRNA axonal transport and local up-regulation could contribute to the hyperexcitability of peripheral nerves in some neuropathic pain states.
Conclusion: Cuff entrapment injury resulted in significantly elevated axonal excitability and increased NaV1.8 immunoreactivity in rat sciatic nerves. The concomitant axonal accumulation of NaV1.8 mRNA may play a role in the pathogenesis of this model of neuropathic pain.
Figures


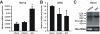
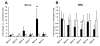
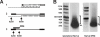
References
-
- IASP Task Force on Taxonomy . Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. IASP Press, Seattle; 1994.
-
- Dong XW, Goregoaker S, Engler H, Zhou X, Mark L, Crona J, Terry R, Hunter J, Priestley T. Small interfering RNA-mediated selective knockdown of Nav1.8 tetrodotoxin-resistant sodium channel reverses mechanical allodynia in neuropathic rats. Neuroscience. 2007;146:812–821. doi: 10.1016/j.neuroscience.2007.01.054. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

