Coordinated activation of the origin licensing factor CDC6 and CDK2 in resting human fibroblasts expressing SV40 small T antigen and cyclin E
- PMID: 19321444
- PMCID: PMC2682861
- DOI: 10.1074/jbc.M900687200
Coordinated activation of the origin licensing factor CDC6 and CDK2 in resting human fibroblasts expressing SV40 small T antigen and cyclin E
Abstract
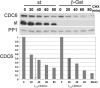
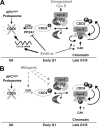
We have previously shown that SV40 small t antigen (st) cooperates with deregulated cyclin E to activate CDK2 and bypass quiescence in normal human fibroblasts (NHF). Here we show that st expression in serum-starved and density-arrested NHF specifically induces up-regulation and loading of CDC6 onto chromatin. Coexpression of cyclin E results in further accumulation of CDC6 onto chromatin concomitantly with phosphorylation of CDK2 on Thr-160 and CDC6 on Ser-54. Investigation of the mechanism leading to CDC6 accumulation and chromatin loading indicates that st is a potent inducer of cdc6 mRNA expression and increases CDC6 protein stability. We also show that CDC6 expression in quiescent NHF efficiently promotes cyclin E loading onto chromatin, but it is not sufficient to activate CDK2. Moreover, we show that CDC6 expression is linked to phosphorylation of the activating T loop of CDK2 in serum-starved NHF stimulated with mitogens or ectopically expressing cyclin E and st. Our data suggest a model where the combination of st and deregulated cyclin E result in cooperative and coordinated activation of both an essential origin licensing factor, CDC6, and an activity required for origin firing, CDK2, resulting in progression from quiescence to S phase.
Figures






References
-
- Malumbres, M., and Barbacid, M. (2005) Trends Biochem. Sci. 30 630–641 - PubMed
-
- Graña, X., Garriga, J., and Mayol, X. (1998) Oncogene 17 3365–3383 - PubMed
-
- Mailand, N., and Diffley, J. F. (2005) Cell 122 915–926 - PubMed
-
- Geng, Y., Yu, Q., Sicinska, E., Das, M., Schneider, J. E., Bhattacharya, S., Rideout, W. M., Bronson, R. T., Gardner, H., and Sicinski, P. (2003) Cell 114 431–443 - PubMed
-
- Geng, Y., Lee, Y. M., Welcker, M., Swanger, J., Zagozdzon, A., Winer, J. D., Roberts, J. M., Kaldis, P., Clurman, B. E., and Sicinski, P. (2007) Mol. Cell 25 127–139 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

