Regulation of the steroidogenic acute regulatory protein gene expression: present and future perspectives
- PMID: 19321517
- PMCID: PMC2676994
- DOI: 10.1093/molehr/gap025
Regulation of the steroidogenic acute regulatory protein gene expression: present and future perspectives
Abstract
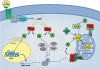
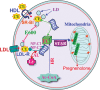
Steroid hormones are synthesized in the adrenal gland, gonads, placenta and brain and are critical for normal reproductive function and bodily homeostasis. The steroidogenic acute regulatory (StAR) protein regulates the rate-limiting step in steroid biosynthesis, i.e. the delivery of cholesterol from the outer to the inner mitochondrial membrane. The expression of the StAR protein is predominantly regulated by cAMP-dependent mechanisms in the adrenal and gonads. Whereas StAR plays an indispensable role in the regulation of steroid biosynthesis, a complete understanding of the regulation of its expression and function in steroidogenesis is not available. It has become clear that the regulation of StAR gene expression is a complex process that involves the interaction of a diversity of hormones and multiple signaling pathways that coordinate the cooperation and interaction of transcriptional machinery, as well as a number of post-transcriptional mechanisms that govern mRNA and protein expression. However, information is lacking on how the StAR gene is regulated in vivo such that it is expressed at appropriate times during development and is confined to the steroidogenic cells. Thus, it is not surprising that the precise mechanism involved in the regulation of StAR gene has not yet been established, which is the key to understanding the regulation of steroidogenesis in the context of both male and female development and function.
Figures

References
-
- Affaitati A, Cardone L, de Cristofaro T, Carlucci A, Ginsberg MD, Varrone S, Gottesman ME, Avvedimento EV, Feliciello A. Essential role of A-kinase anchor protein 121 for cAMP signaling to mitochondria. J Biol Chem. 2003;278:4286–4294. - PubMed
-
- Alberta JA, Epstein LF, Pon LA, Orme-Johnson NR. Mitochondrial localization of a phosphoprotein that rapidly accumulates in adrenal cortex cells exposed to adrenocorticotropic hormone or to cAMP. J Biol Chem. 1989;264:2368–2372. - PubMed
-
- Alpy F, Tomasetto C. Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. J Cell Sci. 2005;118:2791–2801. - PubMed
-
- Arakane F, Sugawara T, Nishino H, Liu Z, Holt JA, Pain D, Stocco DM, Miller WL, Strauss JF., III Steroidogenic acute regulatory protein (StAR) retains activity in the absence of its mitochondrial import sequence: implications for the mechanism of StAR action. Proc Natl Acad Sci USA. 1996;93:13731–13736. - PMC - PubMed

