Comparison of human and rhesus macaque T-cell responses elicited by boosting with NYVAC encoding human immunodeficiency virus type 1 clade C immunogens
- PMID: 19321612
- PMCID: PMC2681993
- DOI: 10.1128/JVI.02345-08
Comparison of human and rhesus macaque T-cell responses elicited by boosting with NYVAC encoding human immunodeficiency virus type 1 clade C immunogens
Abstract
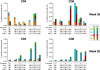
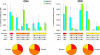
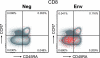
Rhesus macaques (Macaca mulatta) have played a valuable role in the development of human immunodeficiency virus (HIV) vaccine candidates prior to human clinical trials. However, changes and/or improvements in immunogen quality in the good manufacturing practice (GMP) process or changes in adjuvants, schedule, route, dose, or readouts have compromised the direct comparison of T-cell responses between species. Here we report a comparative study in which T-cell responses from humans and macaques to HIV type 1 antigens (Gag, Pol, Nef, and Env) were induced by the same vaccine batches prepared under GMP and administered according to the same schedules in the absence and presence of priming. Priming with DNA (humans and macaques) or alphavirus (macaques) and boosting with NYVAC induced robust and broad antigen-specific responses, with highly similar Env-specific gamma interferon (IFN-gamma) enzyme-linked immunospot assay responses in rhesus monkeys and human volunteers. Persistent cytokine responses of antigen-specific CD4(+) and CD8(+) T cells of the central memory as well as the effector memory phenotype, capable of simultaneously eliciting multiple cytokines (IFN-gamma, interleukin 2, and tumor necrosis factor alpha), were induced. Responses were highly similar in humans and primates, confirming earlier data indicating that priming is essential for inducing robust NYVAC-boosted IFN-gamma T-cell responses. While significant similarities were observed in Env-specific responses in both species, differences were also observed with respect to responses to other HIV antigens. Future studies with other vaccines using identical lots, immunization schedules, and readouts will establish a broader data set of species similarities and differences with which increased confidence in predicting human responses may be achieved.
Figures



References
-
- Amara, R. R., C. Ibegbu, F. Villinger, D. C. Montefiori, S. Sharma, P. Nigam, Y. Xu, H. M. McClure, and H. L. Robinson. 2005. Studies using a viral challenge and CD8 T cell depletions on the roles of cellular and humoral immunity in the control of an SHIV-89.6P challenge in DNA/MVA-vaccinated macaques. Virology 343246-255. - PubMed
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 29269-74. - PubMed
-
- Balla-Jhagjhoorsingh, S. S., G. Koopman, P. Mooij, W. Koornstra, S. McCormack, J. Weber, G. Pantaleo, and J. L. Heeney. 2004. Long-term persistence of HIV-1 vaccine-induced CD4+CD45RA−CD62L−CCR7− memory T-helper cells. AIDS 18837-848. - PubMed
-
- Bart, P. A., R. Goodall, T. Barber, A. Harari, A. Guimaraes-Walker, M. Khonkarly, N. C. Sheppard, Y. Bangala, M. J. Frachette, R. Wagner, P. Liljestrom, J. P. Kraehenbuhl, M. Girard, J. Goudsmit, M. Esteban, J. Heeney, Q. Sattentau, S. McCormack, A. Babiker, G. Pantaleo, and J. Weber. 2008. EV01: A phase I trial in healthy HIV negative volunteers to evaluate a clade C HIV vaccine, NYVAC-C undertaken by the EuroVacc Consortium. Vaccine 263153-3161. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

