Widespread phosphorylation of histone H2AX by species C adenovirus infection requires viral DNA replication
- PMID: 19321613
- PMCID: PMC2687384
- DOI: 10.1128/JVI.00091-09
Widespread phosphorylation of histone H2AX by species C adenovirus infection requires viral DNA replication
Abstract
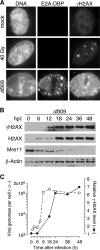
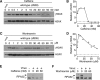
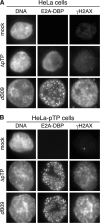
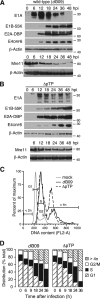
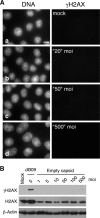
Adenovirus infection activates cellular DNA damage response and repair pathways. Viral proteins that are synthesized before viral DNA replication prevent recognition of viral genomes as a substrate for DNA repair by targeting members of the sensor complex composed of Mre11/Rad50/NBS1 for degradation and relocalization, as well as targeting the effector protein DNA ligase IV. Despite inactivation of these cellular sensor and effector proteins, infection results in high levels of histone 2AX phosphorylation, or gammaH2AX. Although phosphorylated H2AX is a characteristic marker of double-stranded DNA breaks, this modification was widely distributed throughout the nucleus of infected cells and was coincident with the bulk of cellular DNA. H2AX phosphorylation occurred after the onset of viral DNA replication and after the degradation of Mre11. Experiments with inhibitors of the serine-threonine kinases ataxia telangiectasia mutated (ATM), AT- and Rad3-related (ATR), and DNA protein kinase (DNA-PK), the kinases responsible for H2AX phosphorylation, indicate that H2AX may be phosphorylated by ATR during a wild-type adenovirus infection, with some contribution from ATM and DNA-PK. Viral DNA replication appears to be the stimulus for this phosphorylation event, since infection with a nonreplicating virus did not elicit phosphorylation of H2AX. Infected cells also responded to high levels of input viral DNA by localized phosphorylation of H2AX. These results are consistent with a model in which adenovirus-infected cells sense and respond to both incoming viral DNA and viral DNA replication.
Figures

References
-
- Andreassen, P. R., G. P. Ho, and A. D. D'Andrea. 2006. DNA damage responses and their many interactions with the replication fork. Carcinogenesis 27883-892. - PubMed
-
- Bakkenist, C. J., and M. B. Kastan. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421499-506. - PubMed
-
- Bassing, C. H., K. F. Chua, J. Sekiguchi, H. Suh, S. R. Whitlow, J. C. Fleming, B. C. Monroe, D. N. Ciccone, C. Yan, K. Vlasakova, D. M. Livingston, D. O. Ferguson, R. Scully, and F. W. Alt. 2002. Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proc. Natl. Acad. Sci. USA 998173-8178. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

