Visual modulation of auditory responses in the owl inferior colliculus
- PMID: 19321633
- PMCID: PMC2694124
- DOI: 10.1152/jn.91313.2008
Visual modulation of auditory responses in the owl inferior colliculus
Abstract
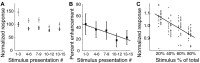
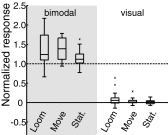
The barn owl's central auditory system creates a map of auditory space in the external nucleus of the inferior colliculus (ICX). Although the crucial role visual experience plays in the formation and maintenance of this auditory space map is well established, the mechanism by which vision influences ICX responses remains unclear. Surprisingly, previous experiments have found that in the absence of extensive pharmacological manipulation, visual stimuli do not drive neural responses in the ICX. Here we investigated the influence of dynamic visual stimuli on auditory responses in the ICX. We show that a salient visual stimulus, when coincident with an auditory stimulus, can modulate auditory responses in the ICX even though the same visual stimulus may elicit no neural responses when presented alone. For each ICX neuron, the most effective auditory and visual stimuli were located in the same region of space. In addition, the magnitude of the visual modulation of auditory responses was dependent on the context of the stimulus presentation with novel visual stimuli eliciting consistently larger response modulations than frequently presented visual stimuli. Thus the visual modulation of ICX responses is dependent on the characteristics of the visual stimulus as well as on the spatial and temporal correspondence of the auditory and visual stimuli. These results demonstrate moment-to-moment visual enhancements of auditory responsiveness that, in the short-term, increase auditory responses to salient bimodal stimuli and in the long-term could serve to instruct the adaptive auditory plasticity necessary to maintain accurate auditory orienting behavior.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

