GABAC receptor-mediated inhibition is altered but not eliminated in the superior colliculus of GABAC rho1 knockout mice
- PMID: 19321639
- PMCID: PMC3817272
- DOI: 10.1152/jn.91001.2008
GABAC receptor-mediated inhibition is altered but not eliminated in the superior colliculus of GABAC rho1 knockout mice
Abstract
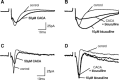
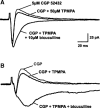
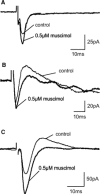
GABA(C) receptors (GABA(C)Rs) are widely expressed in the mammalian subcortical visual system, particularly in the retina and superior colliculus (SC). GABA(C)Rs are composed of specific rho1-3 subunits the expression of which varies among visual structures. Thus rho1 subunits are most abundant in retina, and their loss eliminates GABA(C)R expression and function. In the SC, rho2 subunit expression may be equal to or stronger than rho1 subunit expression; however, results across studies vary considerably. To more directly assess the expression of GABA(C)R subunits, we characterized inhibition in the SC of wild-type (WT) and GABA(C) rho1 Null mice that lack expression of GABA(C) rho1 subunits. We used whole cell patch-clamp recordings and evaluated GABA(C)R-mediated modulation of electrically evoked post synaptic currents using either agonists or antagonists in WT mice. In GABA(C) rho1 Null stratum griseum superficiale (SGS) cells, inhibitory postsynaptic currents were shorter in duration and their excitatory postsynaptic currents (EPSCs) were longer, indicating that a slow GABA(C)R-mediated inhibitory component was reduced in each case. In contrast to retina, GABA(C)R-mediated currents in the SC were altered but not eliminated in GABA(C) rho1 Null mice. In the majority of SC cells in GABA(C) rho1 Null mice, GABA(C)R activation could still be induced to alter EPSC peak amplitudes in putative interneurons and in many projection neurons. These results, compared with previously published data, indicate a fundamental difference between retina and SC in the control of GABA(C)R expression and subunit composition.
Figures







Similar articles
-
Postnatal maturation of GABA(A) and GABA(C) receptor function in the mammalian superior colliculus.Eur J Neurosci. 2001 Oct;14(8):1185-93. doi: 10.1046/j.0953-816x.2001.01746.x. Eur J Neurosci. 2001. PMID: 11703447
-
Disinhibition in rat superior colliculus mediated by GABAc receptors.J Neurosci. 2001 Jan 15;21(2):691-9. doi: 10.1523/JNEUROSCI.21-02-00691.2001. J Neurosci. 2001. PMID: 11160448 Free PMC article.
-
GABAC receptor subunit mRNA expression in the rat superior colliculus is regulated by calcium channels, neurotrophins, and GABAC receptor activity.Brain Cell Biol. 2006 Dec;35(4-6):251-66. doi: 10.1007/s11068-008-9020-0. Epub 2008 Apr 5. Brain Cell Biol. 2006. PMID: 18392729
-
[GABAC receptors: structure and functions].Eksp Klin Farmakol. 2011;74(1):45-9. Eksp Klin Farmakol. 2011. PMID: 21476276 Review. Russian.
-
GABAC receptor-mediated inhibition in the retina.Vision Res. 2004 Dec;44(28):3289-96. doi: 10.1016/j.visres.2004.07.023. Vision Res. 2004. PMID: 15535996 Review.
Cited by
-
GABA-ρ receptors: distinctive functions and molecular pharmacology.Br J Pharmacol. 2017 Jul;174(13):1881-1894. doi: 10.1111/bph.13768. Epub 2017 Apr 12. Br J Pharmacol. 2017. PMID: 28258627 Free PMC article. Review.
-
Neurochemicals for the investigation of GABA(C) receptors.Neurochem Res. 2010 Dec;35(12):1970-7. doi: 10.1007/s11064-010-0271-7. Epub 2010 Oct 21. Neurochem Res. 2010. PMID: 20963487 Review.
-
An Update on GABAρ Receptors.Curr Neuropharmacol. 2010 Dec;8(4):422-33. doi: 10.2174/157015910793358141. Curr Neuropharmacol. 2010. PMID: 21629448 Free PMC article.
-
Modulation of the human ρ1 GABAA receptor by inhibitory steroids.Psychopharmacology (Berl). 2014 Sep;231(17):3467-78. doi: 10.1007/s00213-013-3379-z. Epub 2013 Dec 7. Psychopharmacology (Berl). 2014. PMID: 24317445 Free PMC article.
-
Receptor targets of amacrine cells.Vis Neurosci. 2012 Jan;29(1):11-29. doi: 10.1017/S0952523812000028. Vis Neurosci. 2012. PMID: 22310370 Free PMC article. Review.
References
-
- BollerMBoller M, Schmidt M. Postnatal maturation of GABAA and GABAC receptor function in the mammalian superior colliculus. Eur J Neurosci 14: 1185–1193, 2001. - PubMed
-
- BollerMBoller M, Schmidt M. GABAC receptors in the rat superior colliculus and pretectum participate in synaptic neurotransmission. J Neurophysiol 89: 2035–2045, 2003. - PubMed
-
- BormannJBormann J. The “ABC” of GABA receptors. Trends Pharmacol Sci 21: 16–19, 2000. - PubMed
-
- BormannJBormann J, Feigenspan A. GABAC receptors. Trends Neurosci 18: 515–519, 1995. - PubMed
-
- Boué-GrabotEBoué-Grabot E, Roudbaraki M, Bascles L, Tramu G, Bloch B, Garret M. Expression of GABA receptor rho subunits in rat brain. J Neurochem 70: 899–907, 1998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

