Diverse actions and target-site selectivity of neonicotinoids: structural insights
- PMID: 19321668
- PMCID: PMC2701451
- DOI: 10.1124/mol.109.055186
Diverse actions and target-site selectivity of neonicotinoids: structural insights
Abstract
The nicotinic acetylcholine receptors (nAChRs) are targets for human and veterinary medicines as well as insecticides. Subtype-selectivity among the diverse nAChR family members is important for medicines targeting particular disorders, and pest-insect selectivity is essential for the development of safer, environmentally acceptable insecticides. Neonicotinoid insecticides selectively targeting insect nAChRs have important applications in crop protection and animal health. Members of this class exhibit strikingly diverse actions on their nAChR targets. Here we review the chemistry and diverse actions of neonicotinoids on insect and mammalian nAChRs. Electrophysiological studies on native nAChRs and on wild-type and mutagenized recombinant nAChRs have shown that basic residues particular to loop D of insect nAChRs are likely to interact electrostatically with the nitro group of neonicotinoids. In 2008, the crystal structures were published showing neonicotinoids docking into the acetylcholine binding site of molluscan acetylcholine binding proteins with homology to the ligand binding domain (LBD) of nAChRs. The crystal structures showed that 1) glutamine in loop D, corresponding to the basic residues of insect nAChRs, hydrogen bonds with the NO(2) group of imidacloprid and 2) neonicotinoid-unique stacking and CH-pi bonds at the LBD. A neonicotinoid-resistant strain obtained by laboratory-screening has been found to result from target site mutations, and possible reasons for this are also suggested by the crystal structures. The prospects of designing neonicotinoids that are safe not only for mammals but also for beneficial insects such as honey bees (Apis mellifera) are discussed in terms of interactions with non-alpha nAChR subunits.
Figures




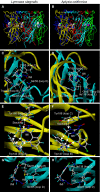
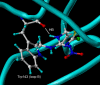


References
-
- Amiri S, Shimomura M, Vijayan R, Nishiwaki H, Akamatsu M, Matsuda K, Jones AK, Sansom MS, Biggin PC, and Sattelle DB (2008) A role for Leu118 of loop E in agonist binding to the α7 nicotinic acetylcholine receptor. Mol Pharmacol 73 1659-1667. - PubMed
-
- Arneric SP, Holladay M, and Williams M (2007) Neuronal nicotinic receptors: a perspective on two decades of drug discovery research. Biochem Pharmacol 74 1092-1101. - PubMed
-
- Bai D, Lummis S, Leicht W, Breer H, and Sattelle D (1991) Actions of imidacloprid and a related nitromethylene on cholinergic receptors of an identified insect motor neurone. Pestic Sci 33 197-204.
-
- Bertrand D, Ballivet M, Gomez M, Bertrand S, Phannavong B, and Gundelfinger ED (1994) Physiological properties of neuronal nicotinic receptors reconstituted from the vertebrate β2 subunit and Drosophila a subunits. Eur J Neurosci 6 869-875. - PubMed
-
- Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Delarue M, and Corringer PJ (2009) X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457 111-114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

