Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behavior of roots
- PMID: 19321713
- PMCID: PMC2675714
- DOI: 10.1104/pp.108.134098
Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behavior of roots
Abstract
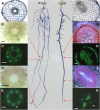
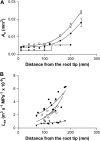
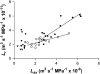
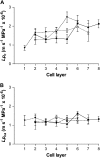
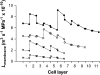
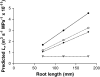
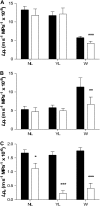
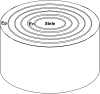
The contrasting hydraulic properties of wheat (Triticum aestivum), narrow-leafed lupin (Lupinus angustifolius), and yellow lupin (Lupinus luteus) roots were identified by integrating measurements of water flow across different structural levels of organization with anatomy and modeling. Anatomy played a major role in root hydraulics, influencing axial conductance (L(ax)) and the distribution of water uptake along the root, with a more localized role for aquaporins (AQPs). Lupin roots had greater L(ax) than wheat roots, due to greater xylem development. L(ax) and root hydraulic conductance (L(r)) were related to each other, such that both variables increased with distance from the root tip in lupin roots. L(ax) and L(r) were constant with distance from the tip in wheat roots. Despite these contrasting behaviors, the hydraulic conductivity of root cells (Lp(c)) was similar for all species and increased from the root surface toward the endodermis. Lp(c) was largely controlled by AQPs, as demonstrated by dramatic reductions in Lp(c) by the AQP blocker mercury. Modeling the root as a series of concentric, cylindrical membranes, and the inhibition of AQP activity at the root level, indicated that water flow in lupin roots occurred primarily through the apoplast, without crossing membranes and without the involvement of AQPs. In contrast, water flow across wheat roots crossed mercury-sensitive AQPs in the endodermis, which significantly influenced L(r). This study demonstrates the importance of examining root morphology and anatomy in assessing the role of AQPs in root hydraulics.
Figures

References
-
- Alleva K, Niemietz CM, Sutka M, Maurel C, Parisi M, Tyerman SD, Amodeo G (2006) Plasma membrane of Beta vulgaris storage root shows high water channel activity regulated by cytoplasmic pH and a dual range of calcium concentrations. J Exp Bot 57 609–621 - PubMed
-
- Biela A, Grote K, Otto B, Hoth S, Hedrich R, Kaldenhoff R (1999) The Nicotiana tabacum plasma membrane aquaporin NtAQP1 is mercury-insensitive and permeable for glycerol. Plant J 18 565–570 - PubMed
-
- Bramley H, Turner NC, Turner DW, Tyerman SD (2007. a) Comparison between gradient-dependent hydraulic conductivities of roots using the root pressure probe: the role of pressure propagations and implications for the relative roles of parallel radial pathways. Plant Cell Environ 30 861–874 - PubMed
-
- Bramley H, Turner DW, Tyerman SD, Turner NC (2007. b) Water flow in the roots of crop species: the influence of root structure, aquaporin activity, and waterlogging. Adv Agron 96 133–196
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

